The two-component intranasal vaccine elicits stronger humoral immunity
To investigate the potential enhanced effects of combining the adenovirus vectors and protein-based vaccines in eliciting superior immune responses, we produced an adenovirus vector carrying the intact full-length spike of XBB.1.5 (Ad5XBB.1.5) (Fig. 1a and Extended Data Fig. 1a) and a trimeric XBB.1.5 RBD-derived protein (RBDXBB.1.5-HR) (Fig. 1b). We formulated the two doses of two-component vaccines by mixing Ad5XBB.1.5 with RBDXBB.1.5-HR in different ratios (Fig. 1c and Extended Data Fig. 1b). First, we added buffer to dilute a certain amount of RBDXBB.1.5-HR protein stock solution, adjusting the pH to 7.6 to ensure consistency with the buffer composition of the Ad5XBB.1.5 adenovirus stock solution. Next, we slowly added the adenovirus stock solution to achieve final concentrations of 5 × 1010 virus particles (VPs) ml−1 and 100 μg ml−1, or 1.0 × 1011 VPs ml−1 and 200 μg ml−1. To prevent adenovirus aggregation during vaccine preparation, the adenovirus should be mixed slowly using magnetic stirring while being added (Extended Data Fig. 1b). We characterized the size distribution and infectivity of the adenovirus in the two-component preparation and found that the mixing process did not affect the particle size or infectivity of the adenovirus component (Extended Data Fig. 1c,d). In addition, stability assay results demonstrated that the two-component vaccine can be stored stably at −20 °C, with unchanged infectivity of the adenovirus component and protein antigenic content (Extended Data Fig. 1e).
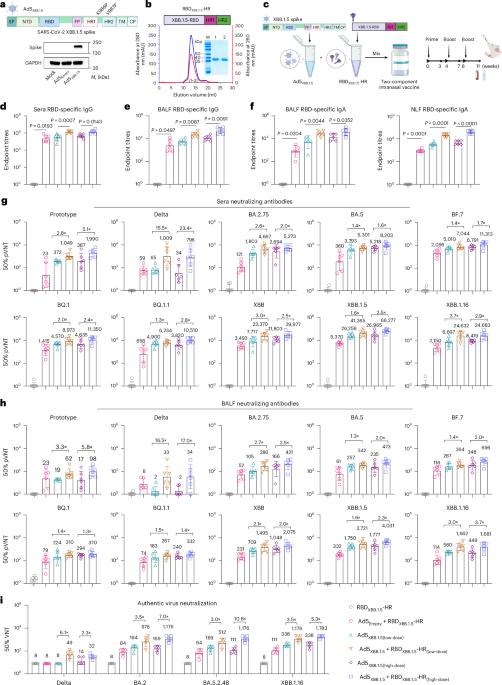
Fig. 1: Intranasal vaccination with the two-component vaccine induces superior humoral immunity compared with the adenoviral vector alone.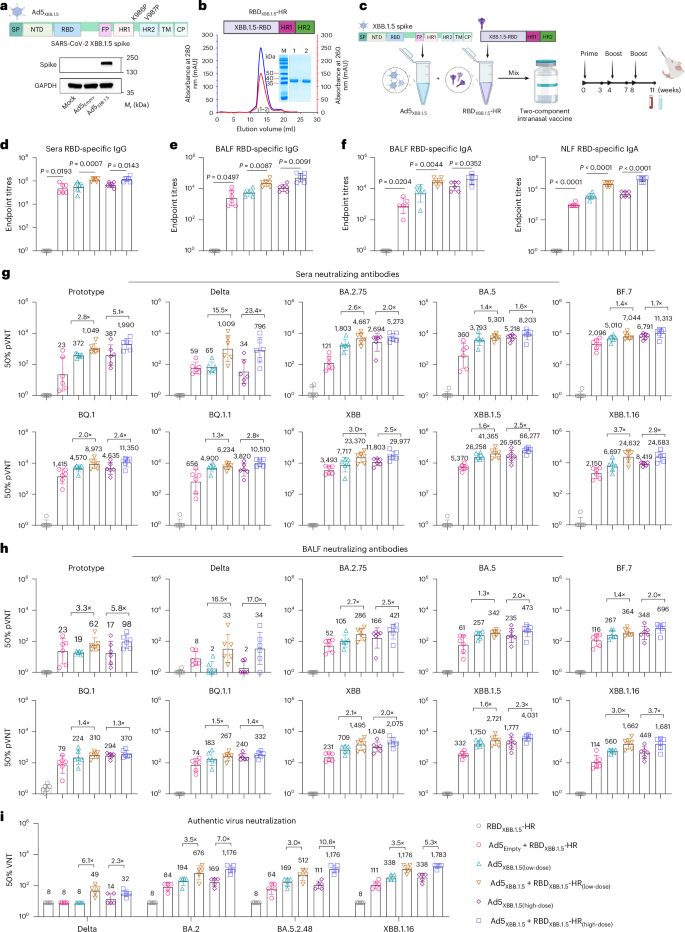
a, Top: schematic representation of the design of full-length XBB.1.5 spike protein-carrying adenovirus. Bottom: western blotting of spike protein expression in A549 cells in the absence or presence of Ad5XBB.1.5. SP, signal peptide; NTD, N-terminal domain; RBD, receptor binding domain; HR1 and HR2, heptad repeats 1 and 2; TM, transmembrane domain; CP, cytoplasmic domain; Mr, relative molecular mass. b, Top: RBDXBB.1.5-HR protein includes an RBD derived from the XBB.1.5 variant, and HR1 and HR2 domains in subunit S2 of spike protein. Bottom: representative elution chromatographs of the recombinant RBDXBB.1.5-HR protein using a calibrated Superdex 200 Increase column. SDS–PAGE analysis of the eluted protein is shown. M, marker; 1, the eluted sample of the ascending part of the protein peak; 2, the eluted sample of the descending part of the peak. Panels a and b are representative of two independent experiments with similar results. mAU, milli absorbance unit. c, Schematic representation of the preparation of the two-component vaccine, immunization and sample collection protocol. BALB/c mice were intranasally immunized with a low dose (2.5 × 109 VPs adenovirus + 5 μg protein) or high dose (5 × 109 VPs adenovirus + 10 μg protein) of the two-component vaccine 3 times at 0, 4 and 8 weeks. Serum and BALF samples in d–i were collected at 11 weeks after the first immunization. d,e, Endpoint titres of anti-RBD IgG in mouse serum (d) and BALF (e) samples (n = 6 mice per group). f, Endpoint titres of anti-RBD IgA in BALF (left) and nasal lavage fluid (NLF) (right) samples (n = 6 mice per group). g,h, Neutralizing antibody titres in serum (g) and BALF (h) samples against Prototype, Delta, BA.2.75, BA.4/5, BF.7, BQ.1, BQ.1.1, XBB, XBB.1.5 and XBB.1.16 pseudoviruses (n = 6 mice per group). pVNT, pseudovirus neutralization titre. i, Sera neutralizing antibody titres against authentic SARS-CoV-2, including Delta, BA.2, BA.5.2.48 and XBB.1.16 (n = 5 mice per group). The numbers above the data points in g–i indicate the GMTs of 50% neutralization for each group, as well as the fold comparisons of GMTs between groups. Data are presented as geometric mean ± s.d. P values in d–f were determined using two-tailed unpaired Student’s t-tests. Schematic illustrations in a–c created with BioRender.com.
We intranasally vaccinated mice with a low dose or high dose of the two-component vaccine containing 2.5 × 109 or 5 × 109 VPs of Ad5XBB.1.5 with 5 μg or 10 μg of RBDXBB.1.5-HR, respectively. Control groups received either naked RBDXBB.1.5-HR or Ad5XBB.1.5 alone. All animals were vaccinated following a prime–boost regimen with a 28-day interval (Fig. 1c). We observed that intranasal delivery of RBDXBB.1.5-HR protein alone hardly induced RBD-specific IgG antibodies in serum samples, while immunization with Ad5XBB.1.5 alone induced a sustained binding antibody response (Fig. 1d). Of note, the combination of Ad5XBB.1.5 and RBDXBB.1.5-HR resulted in a significant increase in the titres of antigen-specific IgG antibodies compared with Ad5XBB.1.5 alone (Fig. 1d), irrespective of the dose. Mucosal antibody response, particularly secretory IgA, plays an important role in mucosal immunity conferred by intranasal COVID-19 vaccines. Recent studies demonstrated that secretory IgA exhibits significantly higher neutralizing potency against Omicron than serum IgG and IgA54,55. Therefore, we further investigated the production of antigen-specific IgG and IgA in the bronchoalveolar lavage fluid (BALF) and nasal lavage fluid from vaccinated mice. Consistent with the results of binding antibodies in sera, the combination of Ad5XBB.1.5 and RBDXBB.1.5-HR induced higher titres of IgG and IgA in the respiratory tract compared with immunization with adenovirus-vectored vaccine alone (Fig. 1e,f). It is worth noting that the endpoint titres of antigen-specific IgG and IgA in the serum and BALF and NLF samples were orders of magnitude higher when RBDXBB.1.5-HR protein was administered with an empty adenovirus vector (Ad5Empty+RBDXBB.1.5-HR), suggesting that the adenovirus vector can improve the immunogenicity of subunit protein and may serve as a favourable adjuvant candidate for protein-based intranasal vaccines (Fig. 1d–f).
We then carried out pseudovirus neutralization assays to evaluate the neutralizing capacities induced by combined spike-carrying adenovirus and RBD-HR proteins. As expected, RBDXBB.1.5-HR protein alone induced only a negligible neutralization response, while the adenovirus-vector vaccine alone induced a relatively strong neutralizing response (Fig. 1g). Notably, the combination of Ad5XBB.1.5 and RBDXBB.1.5-HR remarkably enhanced the cross-neutralization capacities against all types of pseudoviruses. For instance, the geometric mean titres (GMTs) of 50% neutralization in the high-dose Ad5XBB.1.5 group against prototype, Delta and Omicron subvariants BA.2.75, BA.4/5, BF.7, BQ.1, BQ.1.1, XBB, XBB.1.5 and XBB.1.16 were determined to be 387, 34, 2,694, 5,218, 6,791, 4,635, 3,820, 11,803, 26,965 and 8,419, respectively, whereas the GMTs in the combination group of high-dose Ad5XBB.1.5 and RBDXBB.1.5-HR protein were improved by 5.1-, 23.4-, 2.0-, 1.6-, 1.7-, 2.4-, 2.8-, 2.5-, 2.5- and 2.9-fold, respectively. In addition, compared with immunization with low-dose Ad5XBB.1.5 alone, the neutralizing antibody titres in the group receiving the low-dose two-component vaccine increased by 2.8-, 15.5-, 2.6-, 1.4-, 1.4-, 2.0-, 1.3-, 3.0-, 1.6- and 3.7-fold, respectively. Even when comparing the high-dose adenoviral group with the low-dose two-component vaccine group, the latter still exhibited enhanced neutralizing activity to some extent. Similar enhanced effects in neutralization were also observed in the BALF samples (Fig. 1h). It is worth noting that the addition of Ad5Empty also improved the neutralizing antibody titres in sera and respiratory tracts induced by RBDXBB.1.5-HR (Fig. 1g,h). To further confirm the neutralizing potency of antibodies induced by the combination of Ad5XBB.1.5 and RBDXBB.1.5-HR, authentic virus neutralizing assays were performed (Fig. 1i). Combining Ad5XBB.1.5 and RBDXBB.1.5-HR protein exhibited superior neutralization with cross-neutralizing activities against live BA.2 and BA.5.2.48 and XBB.1.16 viruses. Specifically, compared with Ad5XBB.1.5 alone, the GMTs for 50% neutralization against XBB.1.16 in the low-dose group of Ad5XBB.1.5 + RBDXBB.1.5-HR were improved by 3.5-fold, and by 5.3-fold in the high-dose group, and reached 1,176 and 1,783, respectively. The lower doses of adenovirus (5 × 107 VPs and 5 × 108 VPs) combined with RBD-HR protein induce similar superior humoral immune response compared with individual components (Extended Data Fig. 2a–d). These results indicated that the combination of adenovirus and subunit protein antigens can elicit superior humoral immune responses compared with the individual vaccine components.
The two-component vaccine induces superior airway cellular immunity
In addition to IgA antibodies, TRM cells are another dominant component of mucosal immunity, rapidly responding to prevent virus infection. In the next set of experiments, we detected the frequencies of TRM cells in BALF samples after vaccination. Consistent with our expectations, a significant increase in the frequency of antigen-experienced CD8+ TRM (CD44+CD69+CD103+), but not CD4+ TRM cells, was observed when combining the adenovirus and RBD-HR protein (Fig. 2a,b). Enzyme-linked immunospot assay (ELISpot) was employed to determine IFNγ-spot-forming cells (SFCs) in BALF samples after stimulation with full-length spike peptide pools, and the results showed greatest abundance of IFNγ-secreting cells in the group of the two-component vaccine (Fig. 2c). To further ascertain the antigen-specific T cells in the mucosal tissue, we isolated lung tissues processed into a single-cell suspension and subsequently stimulated with spike peptide pools to detect the expression of intracellular IFNγ and TNF cytokines via intracellular cytokine staining (ICS). The combination of Ad5XBB.1.5 and RBDXBB.1.5-HR resulted in increased numbers of spike-specific IFNγ- and TNF-secreting memory T cells, indicating that the two-component vaccine induced more robust T cell responses in the local mucosal environment compared with the adenovirus-vectored vaccine alone (Fig. 2d,e). Further analysis revealed that these increased mucosal TRM cells are spike protein antigen-responsive, as evidenced by their cytokine IFNγ production (antiviral effect) after stimulation with antigens (Extended Data Fig. 3).
Fig. 2: Intranasal immunization with the two-component vaccine elicits superior mucosal and systemic cellular immune responses compared with Ad5XBB.1.5 alone.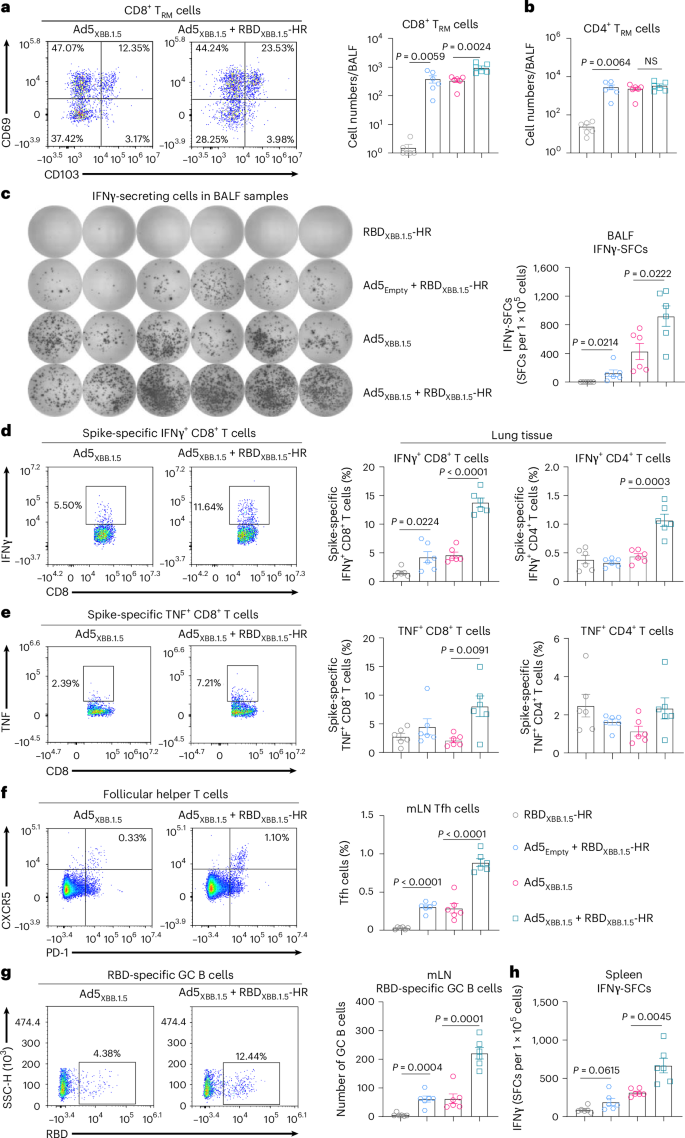
a, Left: representative flow cytometry graphs showing antigen-experienced CD8+ TRM cells induced by Ad5XBB.1.5 or the two-component vaccine. Right: the absolute number of CD8+ TRM cells in BALF analysed by flow cytometry. Antigen-experienced TRM cells were gated on CD44+CD69+CD103+. b, Absolute numbers of antigen-experienced CD4+ TRM cells in BALF. c, ELISpot analysis of IFNγ-SFCs in BALF samples after stimulation with peptide pools of SARS-CoV-2 XBB.1.5 spike. d,e, Representative graphs and quantitative percentages of XBB.1.5 spike-specific IFNγ (d) and TNF (e)-producing memory T cells in lung tissue were analysed after stimulation with XBB.1.5 spike peptide pools. f,g, Frequency of T follicular helper cells (CD4+CXCR5+PD-1+) (f) and RBDXBB.1.5-specific germinal centre B cells (CD19+GL7+CD95+) (g) in mediastinal lymph nodes (mLN). h, IFNγ-SFCs among splenocytes after stimulation with XBB.1.5 spike peptide pools. All tissue samples in Fig. 2, including BALF, lung, mLN and spleen samples, were collected at week 11 after the first immunization. In a–h, n = 6 mice per group; data are presented as mean ± s.e.m. P values between the RBDXBB.1.5-HR and Ad5Empty + RBDXBB.1.5-HR groups, as well as between Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR were calculated using two-tailed unpaired Student’s t-tests. NS, not significant.
Germinal centre B cell (GC B) and T follicular helper (Tfh) cell responses are critical for long-term protective immunity and the formation of memory B cells56,57. Thus, we evaluated the Tfh (CD4+CXCR5+PD-1+) and RBD-specific GC B (CD19+GL7+CD95+) cell responses in mediastinal lymph nodes. As expected, combining adenovirus and protein induced the highest frequencies of Tfh and the largest total number of antigen-specific GC B cells, compared with immunization with Ad5XBB.1.5 alone (Fig. 2f,g). In addition to the stronger mucosal cellular immunity, we also noticed the improved antigen-specific splenocytes in the group receiving the two-component vaccine vs the adenovirus vector alone, suggesting a superior systemic cellular immune response (Fig. 2h). Similar enhancements in cellular immune response can be observed in the lower dose of the adenovirus plus protein antigens (Extended Data Fig. 2e,f). Thus, these results demonstrate that the two-component vaccines, which combine Ad5XBB.1.5 and RBDXBB.1.5-HR proteins, can provide superior mucosal and systemic cellular response against SARS-CoV-2 infection.
The STING pathway is critical for the adenovirus-vector adjuvant effect
It is remarkable that the combination of adenovirus and subunit protein can elicit superior immunity compared with individual components. Even the empty adenovirus vector can serve as an adjuvant to improve the immunogenicity of naked RBD-HR protein owing to its immunomodulatory property. To discover the potential signalling pathways involved in the adjuvant effects of the adenovirus vector, we intranasally administered the two-component vaccine to multiple transgenic mice deficient in signalling pathways associated with host immune response to adenovirus41,43, including Tlr2−/−, Tlr9−/−, Myd88−/−, Nlrp3−/−, Casp1−/−, Il1b−/−, Sting−/− and Cd8a−/− mice, and evaluated the improvements in antibodies induced by the two-component vaccine. Since the XBB lineages were not yet prevalent worldwide during our investigation of the signalling pathways, we utilized a two-component vaccine that combined BA.5 spike-carrying adenovirus (Ad5BA.5) and a BA.5-derived RBD-HR (RBDBA.5-HR) in this experiment (Extended Data Fig. 4a–c). The improvements in RBD-specific antibody titres were still observed in wildtype (WT) and most transgenic mice. However, there was no significant difference between the endpoint titres of sera IgG and BALF IgA antibodies induced by adenovirus alone compared with the two-component vaccines in Sting−/− mice (Fig. 3a,b). To further investigate the mechanism of adjuvant effect exerted by the adenovirus, we immunized the mice with the adenovirus vector without spike antigen (Ad5Empty) along with the RBDBA.5-HR protein. Consistent with the result above, the adjuvant effect of the adenovirus vector on systemic and mucosal humoral immune responses was remarkably impaired in the Sting−/− mice (Fig. 3c,d). Since MYD88 is the most important adaptor protein for TLR-2 and TLR-9 signalling pathways, the Myd88−/− mice were also utilized as controls for the subsequent evaluation of the adjuvant effect of the adenovirus vector on cellular immunity.
Fig. 3: The STING signalling pathway is required for the adenovirus vector-induced adjuvant effect.
a, Endpoint titres of anti-RBD IgG in serum samples from WT or transgenic (Tlr9−/−, Tlr2−/−, Myd88−/−, Nlrp3−/−, Casp1−/−, Il1b−/−, Sting−/−, Cd8a−/−) mice immunized with Ad5BA.5 alone or Ad5BA.5 plus RBDBA.5-HR (n = 6 mice per group). b, Endpoint titres of anti-RBD IgA in BALF samples from WT or Sting−/−mice immunized with Ad5BA.5 alone or Ad5BA.5 plus RBDBA.5-HR (n = 6 mice per group). c, Endpoint titres of sera anti-RBD IgG from WT or transgenic mice immunized with Ad5Empty plus RBDBA.5-HR. WT mice intranasally vaccinated with naked RBDBA.5-HR were used as control (n = 6 mice per group). d, Endpoint titres of BALF IgA from WT or Sting−/−mice immunized with Ad5Empty plus RBDBA.5-HR (n = 6 mice per group). e, Absolute number of antigen-experienced CD8+ TRM cells in BALF from vaccinated WT, Myd88−/− or Sting−/− mice (n = 6 mice per group). f, t-SNE maps were generated by concentrating CD3+ T cells in the BALF from WT or Sting−/− mice vaccinated with Ad5BA.5 plus RBDBA.5-HR, and heat map projections of CD69 and CD103 expression on t-SNE maps are shown (n = 3 mice each group). Analysis was carried out using FlowJo v.10. g,h, Percentage of spike-specific CD8+ (g) and CD4+ (h) memory T cells in lung tissue of vaccinated WT, Myd88−/− or Sting−/− mice (n = 6 mice per group). i, Percentage of antigen-specific IFNγ-producing CD8+ T cells in lung from mice vaccinated with Ad5Empty + RBDBA.5-HR (n = 6 mice per group). Data are presented as geometric mean ± s.d. (a–d), and as mean with s.e.m. (e,g–i). P values were determined using two-tailed unpaired Student’s t-tests (a,b), one-way ANOVA followed by Dunnett’s multiple comparisons test (c) or Tukey’s multiple comparisons test (d), two-way ANOVA followed by Sidak’s multiple comparisons test (e–h), and one-way ANOVA followed by Tukey’s multiple comparisons test (i).
Next, we evaluated the role of STING signalling in the induction of mucosal cellular immunity. As expected, the increase in the number of CD8+ TRM induced by the combination of Ad5BA.5 and RBDBA.5-HR was abrogated in Sting−/− mice (Fig. 3e). We then established t-distributed stochastic neighbour embedding (t-SNE) maps based on pooled CD3+ T cells in BAL fluid from wildtype and Sting−/− mice. Heat maps were generated to visualize the expression intensities of CD69 and CD103, two classic surface markers for TRM cells (Fig. 3f). In wildtype mice, the expressions of CD69 and CD103 in CD3+ T cells were significantly increased after immunization with the two-component vaccine. In contrast, the expression of these two markers was remarkably decreased in Sting−/− mice, suggesting that STING signalling is critical for the induction of mucosal TRM cells. In addition, the enhancements of antigen-specific CD8+ and CD4+ T memory cells were significantly impaired in Sting knockout mice (Fig. 3g,h), and the adjuvant effect of the Ad5Empty on cellular immune response was completely abolished in the Sting−/− mice (Fig. 3i). These results indicate that the STING signalling pathway is crucial for the establishment of adenovirus-adjuvanted cellular immune response.
Single-cell RNA sequencing (scRNA-seq) was conducted to investigate the STING signalling pathway, which influences the immunization efficacy of the two-component vaccine. The cell populations were visualized using a t-SNE map (Extended Data Fig. 5a,b), revealing a significant increase in B memory cells in the lung tissues of wildtype mice following immunization, compared with Sting−/− mice. Gene expression analysis indicated that transcript levels of Tmem173, the gene encoding STING, were predominantly enriched in dendritic cell (DC) populations, including CCR7+ DCs, DC1 and plasmacytoid DCs (pDCs) (Extended Data Fig. 5c,d). Notably, these levels were significantly upregulated after administration of the two-component vaccine (Extended Data Fig. 5e). Next, we focused on the activation of the STING pathway by selecting key genes involved in the signalling cascade, including Adar, Ccl5, Ddx41, Ifi203, Ifit1, Irf7, Irf9, Isg15, Oas1a and Trim21. Our findings showed that this pathway was significantly activated in wildtype mice, whereas it was diminished in STING knockout mice immunized with the two-component vaccine (Extended Data Fig. 5f). This suggests that STING pathway activation in DC populations is crucial for the protective efficacy of the vaccine. Indeed, the expression levels of genes involved in antigen processing and presentation were markedly upregulated in DC populations from wildtype mice after immunization, while these levels were significantly inhibited in Sting−/− mice (Extended Data Fig. 5g). In addition, adenoviral vectors, including Ad5Empty and Ad5XBB.1.5, facilitated protein antigen capture in DCs in vitro, as evidenced by enhanced phagocytosis of fluorescence-conjugated RBDXBB.1.5-HR proteins in DCs in the presence of adenoviral vectors (Extended Data Fig. 5h). Furthermore, assessments of plasma cell formation and B cell activation revealed impaired humoral immunity in Sting−/− mice following immunization, consistent with reduced antibody production (Extended Data Fig. 5i). Therefore, these results demonstrate that the activation of the STING signalling pathway, particularly in antigen-presenting DCs, is imperative for the induction of protective immunity by the combination of adenovirus and protein antigens.
The three-component vaccine enhances cross-neutralizing activity
We have demonstrated that the combination of adenovirus-vectored and protein vaccines (Ad5XBB.1.5 + RBDXBB.1.5-HR, Ad5BA.5 + RBDBA.5-HR) can induce superior protective immunity. To investigate the potential of combining adenovirus and subunit protein antigens as a platform for developing a universal COVID-19 vaccine with broader-spectrum neutralizing activities, we generated a multivalent vaccine by incorporating additional antigens and adjusting component ratios. The goal was to target the transmission of multiple circulating Omicron subvariants. We developed an intranasal three-component vaccine consisting of Ad5XBB.1.5 (5 × 109 VPs), RBDXBB.1.5-HR (7.5 μg) and RBDBA.5-HR (2.5 μg) (Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR) (Fig. 4a). Similar to the two-component vaccines, mice immunized with the three-component vaccine exhibited superior humoral and cellular immune responses compared with those immunized with Ad5XBB.1.5 vaccine alone.
Fig. 4: The three-component vaccine elicits superior humoral and cellular immunity with broad-spectrum neutralization.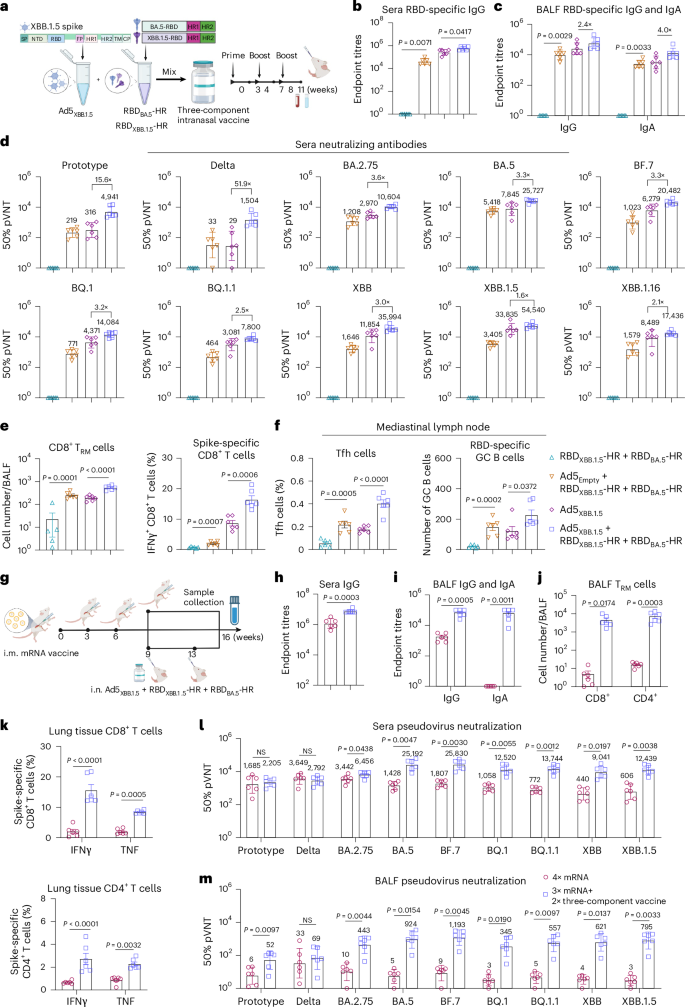
a, Schematic representations of the preparation of a three-component vaccine, consisting of Ad5XBB.1.5 (5 × 109 VPs), RBDXBB.1.5-HR (7.5 μg) and RBDBA.5-HR (2.5 μg). b,c, Endpoint titres of sera anti-RBD IgG (b), and BALF anti-RBD IgG and IgA (c) (n = 6 mice per group). d, Neutralizing antibody titres in sera samples (n = 6 mice per group). e, Absolute number of antigen-experienced CD8+ TRM cells in BALF (left), and the percentage of spike-specific IFNγ-secreting CD8+ T cells in lung tissue (right) from vaccinated mice (n = 6 mice per group). f, Frequency of Tfh cells (left) and the absolute number of RBD-specific GC B cells (right) in mediastinal lymph nodes (n = 6 mice per group). g–m, BALB/c mice were intramuscularly (i.m.) immunized with 3 doses of 5 μg mRNA/50 μl of encapsulated liposome (LPX)/Spike-mRNA, followed by 1 dose of homologous injection of mRNA vaccine (4× mRNA), or twice intranasal (i.n.) delivery of three-component vaccine (3× mRNA + 2× three-component vaccine) (g); n = 6 mice per group. The anti-RBD IgG in sera (h), anti-RBD IgG, IgA antibody (i) and TRM cells in BALF (j), spike-specific T cells in lung tissue (k), and neutralization antibody titres of sera (l) and BALF (m) samples were detected. The numbers above the data points in d, l and m indicate the GMTs of 50% neutralization for each group, as well as the fold comparisons of GMTs between groups in d. Data are presented as geometric mean ± s.d. (b–d,h,i,l,m), and as mean ± s.e.m. (e,f,j,k). P values between the RBDXBB.1.5-HR + RBDBA.5-HR and Ad5Empty + RBDXBB.1.5-HR + RBDBA.5-HR groups, as well as between Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR were determined using two-tailed unpaired Student’s t-tests (b,c,e,f). P values between the homologous and heterologous immunization groups were determined using two-tailed unpaired Student’s t-tests (h,l,m); two-way ANOVA followed by Sidak’s multiple comparisons test was performed for i–k. Schematic in a and schematic illustration in g created with BioRender.com.
The three-component vaccine induced higher levels of binding antibodies in sera and the local respiratory tract (Fig. 4b,c). Pseudovirus neutralization assays further confirmed the improvements in neutralizing capacities (Fig. 4d). It is worth noting that the three-component vaccine further increased the neutralizing activities against other Omicron variants without compromising the neutralization of XBB lineages (Extended Data Fig. 6a). Specifically, the GMTs of 50% neutralization in the high dose of the two-component vaccine group against BA.2.75, BA.5, BF.7, BQ.1 and BQ.1.1 were improved by 2.0-, 1.6-, 1.7-, 2.4- and 2.8-fold, respectively, compared with Ad5XBB.1.5 (Fig. 1g), while the GMTs were improved by 3.6-, 3.3-, 3.3-, 3.2- and 2.5-fold after immunization of the three-component vaccine (Fig. 4d and Extended Data Fig. 6a). In addition, the GMTs in the group that received the three-component vaccine against XBB, XBB.1.5 and XBB.1.16 were also increased by 3.0-, 1.6- and 2.1-fold, respectively, compared with Ad5XBB.1.5 alone.
In addition to the humoral immune response, the three-component vaccine also resulted in increased frequencies of antigen-experienced TRM in BALF samples and IFNγ-secreting memory CD8+ T cells in lung tissues (Fig. 4e). Furthermore, Tfh and RBD-specific GC B cells in the mediastinal lymph node were significantly increased in the three-component vaccine group (Fig. 4f). Two additional three-component vaccines were prepared and evaluated, featuring adjusted RBDXBB.1.5-HR and RBDBA.5-HR antigen ratios of 1:1 and 1:3 (Extended Data Fig. 6b). Both vaccines elicited comparable cross-neutralization responses, with the neutralizing activity against pre-XBB subvariants progressively enhanced by increasing the proportion of RBDBA.5-HR protein antigen in the intranasal formulation. Notably, the intranasal vaccine with a 3:1 antigen ratio of RBDXBB.1.5-HR to RBDBA.5-HR demonstrated the strongest neutralization against XBB lineages, prompting its selection for further studies. These results strongly demonstrate that combining adenovirus-vectored and subunit protein antigens can be used as an intranasal vaccine platform. Moreover, multivalent vaccines can be developed by adjusting the proportions of different components in the vaccine, allowing for the elicitation of broader-spectrum neutralizing capacities against multiple circulating Omicron subvariants.
The three-component vaccine as a heterologous booster to improve immunity
The messenger RNA-based COVID-19 vaccines have been widely used around the world6. Therefore, we investigated whether the three-component vaccine could serve as a heterologous booster in sequential immunization. Animals received three intramuscular injections of an mRNA vaccine on days 0, 21 and 42 (ref. 52), followed by a two-dose intranasal immunization with the three-component vaccine (protein components usually work better after two immunizations) (Fig. 4g). As expected, heterologous immunization with the three-component vaccine significantly improved the titres of RBD-specific IgG (Fig. 4h) and neutralizing antibodies (Fig. 4l) in the sera compared with homologous vaccination with mRNA vaccines. Consistent with previous studies, the circulating Omicron subvariants, particularly the XBB lineages, exhibited extensive resistance to neutralization by mRNA vaccines. However, intranasal delivery of the three-component vaccine rescued the compromised neutralizing activities against Omicron subvariants that included the XBB lineages (Fig. 4l). Remarkably, the GMTs of 50% sera neutralization in the heterologous vaccination group against BA.5, BF.7, BQ.1, BQ.1.1, XBB and XBB.1.5 subvariants were improved by 17.6-, 14.3-, 11.8-, 17.8-, 20.5- and 20.5- fold, respectively, compared with the homologous vaccination group.
In addition to enhancing systemic immune response, heterologous intranasal immunization with the three-component vaccine conferred additional respiratory mucosal immunity, characterized by higher levels of antigen-specific IgA and IgG in BALF (Fig. 4i), increased frequencies of TRM cells in BALF and lung tissue (Fig. 4j and Extended Data Fig. 6c), elevated Tfh and GC B cells in mediastinal lymph nodes (Extended Data Fig. 6d,e), and abundant spike-specific T memory cells in lung tissues (Fig. 4k). Unlike homologous immunization with mRNA vaccine, the heterologous immunization regimen resulted in high levels of neutralizing antibodies in the respiratory tract against all variants in the panel (Fig. 4m). The GMT of 50% neutralization against XBB and XBB.1.5 variants in BALF reached 621 and 795, respectively, indicating potent neutralizing activity against the circulating XBB lineages. Remarkably, the antibody responses in the blood and mucosal immunity were apparently elevated in a quicker way, even with only one dose of the three-component vaccine following three injections of the mRNA vaccine (Extended Data Fig. 6f–k). These findings indicate that the combination of adenovirus and protein antigens can be a promising candidate for heterologous immunization after mRNA vaccine administration.
The two-component vaccine protects against viral infection and transmission
Due to the existence of imprinted immunity in humans with a hybrid immune background, the US FDA has recommended the use of the component derived from the XBB descendant lineage as the vaccine antigen. Therefore, we next use the two-component (Ad5XBB.1.5 + RBDXBB.1.5-HR) rather than the three-component (Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR) intranasal vaccine for virus challenge assay and clinical trial.
To evaluate the efficacy of the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine against the XBB.1.16 variant in vivo, we conducted a live virus challenge assay. Mice were intranasally immunized with three doses of Ad5Empty + RBDXBB.1.5-HR, Ad5XBB.1.5 or Ad5XBB.1.5 + RBDXBB.1.5-HR, and challenged with 1 × 106 plaque-forming units (p.f.u.s) of XBB.1.16 Omicron via the intranasal route (Fig. 5a). Mice administered with PBS and naked RBDXBB.1.5-HR served as controls. Changes in viral loads of throat swab samples were monitored daily using quantitative PCR with reverse transcription (RT–qPCR). Throughout the experiment, a large amount of viral load was detected in throat swab samples from mice administered with PBS and RBDXBB.1.5-HR, while immunization with Ad5Empty + RBDXBB.1.5-HR, Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR markedly reduced the viral loads in throat swabs (Fig. 5b). Of note, on day 4 post infection, the group receiving Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine had the lowest viral loads with almost undetectable genomic RNA.
Fig. 5: The two-component intranasal vaccine effectively protects against live viral infection and prevents viral transmission.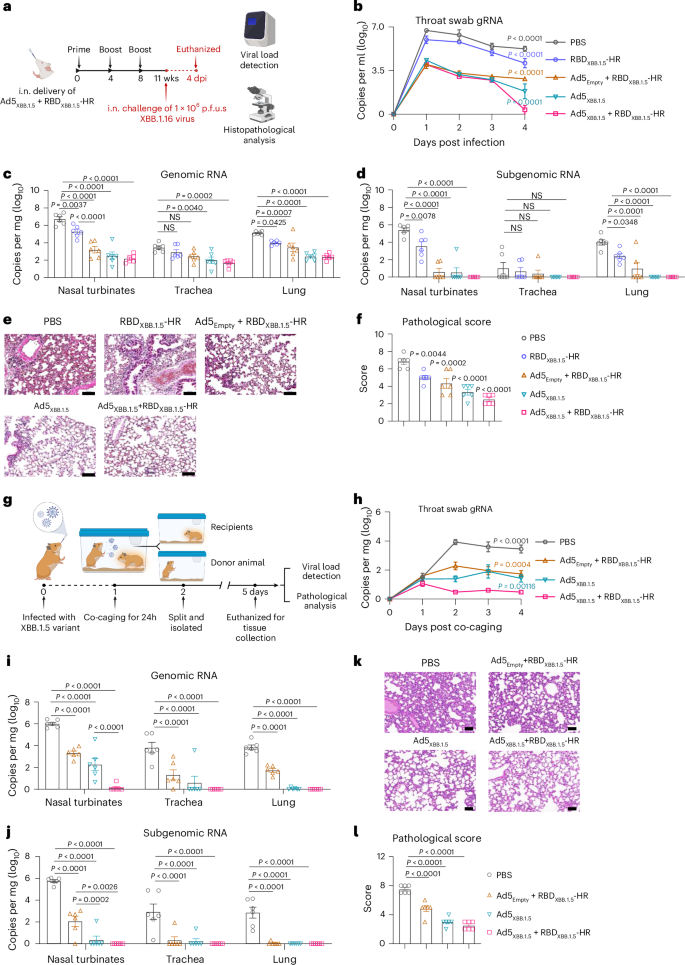
a, BALB/c mice were intranasally immunized three times with Ad5XBB.1.5 alone and two-component (Ad5XBB.1.5 + RBDXBB.1.5-HR) vaccines. Mice treated with PBS, naked RBDXBB.1.5-HR and Ad5Empty plus RBDXBB.1.5-HR were used as controls. Immunized BALB/c mice were challenged with 1 × 106 p.f.u.s of live SARS-CoV-2 XBB.1.16 Omicron viruses. Wks, weeks. b, Changes in viral loads of throat swabs post SARS-CoV-2 infection were determined. c,d, Nasal turbinates, trachea and lung tissue were collected on day 4 post infection to determine the levels of gRNA (c) and sgRNA (d). e,f, Representative histopathological images (e) and corresponding pathological scores (f) of lung tissues from mice challenged with Omicron. n = 6 mice per group (b–d,f). g, Schematic illustration of the contact/airborne transmission protection experiment. Syrian golden hamsters were first intranasally inoculated with 1 × 104 p.f.u.s of XBB.1.5 variants and placed in a cage with four recipient groups that had previously received PBS, Ad5Empty + RBDXBB.1.5-HR, Ad5XBB.1.5 or Ad5XBB.1.5 + RBDXBB.1.5-HR vaccines. After 24 h of co-caging, they were separated into individual cages. On day 4 post exposure, all hamsters were killed for tissue collection. h–j, The gRNA levels in throat swabs (h) and respiratory tissues (i), and sgRNA levels in tissues (j) were determined. k,l, Representative histopathological images (k) and corresponding pathological scores (l) of lung tissues from hamsters post infection. n = 6 hamsters per group (h–j,l). Scale bars, 100 μm (e,k). Data are presented as mean ± s.e.m. P values were determined using two-way ANOVA followed by Tukey’s multiple comparisons test after logarithmic transformation (b–d,h–j), and one-way ANOVA followed by Dunnett’s multiple comparisons test (f,l). The coloured P values in b and h correspond to the comparison between each group and the two-component intranasal vaccine group. Schematic illustrations in a and g created with BioRender.com.
The viral loads in multiple tissues of the respiratory system were detected on day 4 post infection. As expected, the Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR vaccines provided significant reductions in gRNA levels in the upper and lower respiratory tracts, including the nasal turbinates, trachea and lung tissues (Fig. 5c). Even the RBDXBB.1.5-HR protein mixed with empty adenovirus vector (Ad5Empty+RBDXBB.1.5-HR) notably reduced the viral burden in respiratory tracts. To further evaluate the protective effects conferred by our two-component vaccine, we tested the levels of viral subgenomic RNA (E gene) in tissues, which serves as an indicator of active viral replication. High levels of sgRNA were detected in the collected samples in PBS (geometric mean copy numbers of E gene per mg tissue: turbinate, 2.52 × 105; trachea, 10.3; lung, 9.64 × 103) and RBDXBB.1.5-HR (turbinate, 3.45 × 103; trachea, 4.60; lung, 2.51 × 102). Consistent with the gRNA results, the levels of sgRNA were significantly decreased in the Ad5Empty + RBDXBB.1.5-HR and Ad5XBB.1.5 groups, with undetectable sgRNA in the majority of samples. However, a small number of tissues still exhibited active viral replication in these two groups (one nasal turbinate sample in the Ad5XBB.1.5 group, two nasal turbinate samples, one trachea and two lung tissue samples in the Ad5Empty + RBDXBB.1.5-HR group). In contrast to the other groups, intranasal delivery of Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine was more effective in preventing virus replication in the upper and lower respiratory tracts, as evidenced by undetectable sgRNA in all samples from mice immunized with Ad5XBB.1.5 + RBD XBB.1.5-HR (Fig. 5d).
Moreover, the effective protection conferred by the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine correlated with a remarkable reduction in lung pathology. Histopathological evaluation revealed mild pathologic changes in lung tissue from mice in the PBS and RBDXBB.1.5-HR protein groups, characterized by multifocal areas of consolidation, mild alveolar septa thickening, alveolar congestion and small patches of inflammation (Fig. 5e,f). AdEmpty + RBDXBB.1.5-HR partially alleviated the pathological changes. Notably, no evident pathological changes were observed in the lung tissues collected from mice immunized with Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR vaccines, with all pathological scores reduced.
Besides using direct intranasal instillation of live viruses for the viral challenge, the ‘contact/airborne’ infection model was employed to evaluate the efficacy of the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine in preventing viral host-to-host transmission (Fig. 5g). In this model, a group of Syrian golden hamsters was first intranasally inoculated with 1 × 104 p.f.u.s of live XBB.1.5 variants and designated as ‘donor animals’. At 1 day post infection, the donor animals were placed in a cage with four recipient groups that had previously received PBS, Ad5Empty + RBDXBB.1.5-HR, Ad5XBB.1.5 or Ad5XBB.1.5 + RBDXBB.1.5-HR vaccines, sharing diet, bedding and air. After 24 h of co-caging, the donor and recipient animals were separated into individual cages, and viral loads from throat swab samples were monitored daily throughout the study. On day 4 post exposure to the donor, all hamsters were killed, and various respiratory tissues were collected for viral load detection and histopathological examination. Consistent with findings in mice, all animals that had received intranasal immunization demonstrated both viral transmission and reduced lung pathological changes (Fig. 5h–l). Notably, the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine resulted in the lowest viral burden in throat swab samples (Fig. 5h) and respiratory tissues, particularly in the nasal turbinates (Fig. 5i). In the group receiving Ad5Empty + RBDXBB.1.5-HR, five nasal turbinate samples and one trachea sample showed active viral replication with high levels of sgRNA, and one nasal turbinate and one trachea sample exhibited detectable sgRNA in the group receiving Ad5XBB.1.5, while all samples from the Ad5XBB.1.5 + RBDXBB.1.5-HR group showed undetectable sgRNA (Fig. 5j). These results strongly indicate that the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine is an effective intranasal vaccine candidate for protecting against viral challenges and transmission of Omicron sublineages in vivo.
The two-component vaccine improves systemic and mucosal immunity in humans
To further investigate the tolerability and immunogenicity of this intranasal two-component vaccine in the clinic, we conducted a clinical investigator-initiated trial (IIT), and 70 human participants were recruited (Registration number ChiCTR2300069022). All participants in this study provided written informed consent, and the trial was performed according to the principles of the Biomedical Ethics Review Committee of West China Hospital. Adults 18 years and older with a documented history of 2 or 3 doses of the COVID-19 vaccine and with more than 3 months after the last vaccination were eligible for enrolment. In addition, the participants were advised to undergo detection of SARS-CoV-2 antigen within the study to rule out elevated antibody response caused by infection. Most participants received 2 or 3 doses of an inactivated virus vaccine and experienced a BA.5 infection wave from December 2022 to January 2023 in mainland China58. Since then, they have not been infected, and had a 6-month interval between the infection and the vaccination. Therefore, it is likely that most participants were not infected with XBB subvariants. Detailed information about individual participants, including their vaccination and infection histories, can be found in Supplementary Table 1.
The first objective of this investigation was to evaluate the tolerability and safety of the intranasal two-component vaccine. Seventy human participants were assigned to two groups, receiving 2 doses of the two-component vaccine. The first group received 1 × 1010 VPs of Ad5XBB.1.5 plus 20 μg of RBDXBB.1.5-HR protein (comprising 20 participants), while the second group received 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR (comprising 50 participants). The immunization schedule follows a prime–boost regimen spaced 14 days apart (Fig. 6a). In general, there were no significant differences in adverse reactions between these two dosage groups. The most common adverse reactions were predominantly mild (grades 1 and 2) and were transient and self-limiting, and total solicited adverse reactions were reported by 7.14% of all 70 human participants, with local symptoms including nasal obstruction (2.86%), sore throat (1.43%), nasal discharge (1.43%) and systemic symptoms of headache (4.29%) (Fig. 6b). The frequency and severity of solicited adverse events were not related to the dose level. In addition, total unsolicited adverse events within 30 days of vaccination were reported by 1.43% of all participants. No adverse reaction of grade Ⅲ and serious adverse events occurred in the trial.
Fig. 6: Intranasal delivery of two-component vaccine improves systemic and mucosal immune responses in human participants.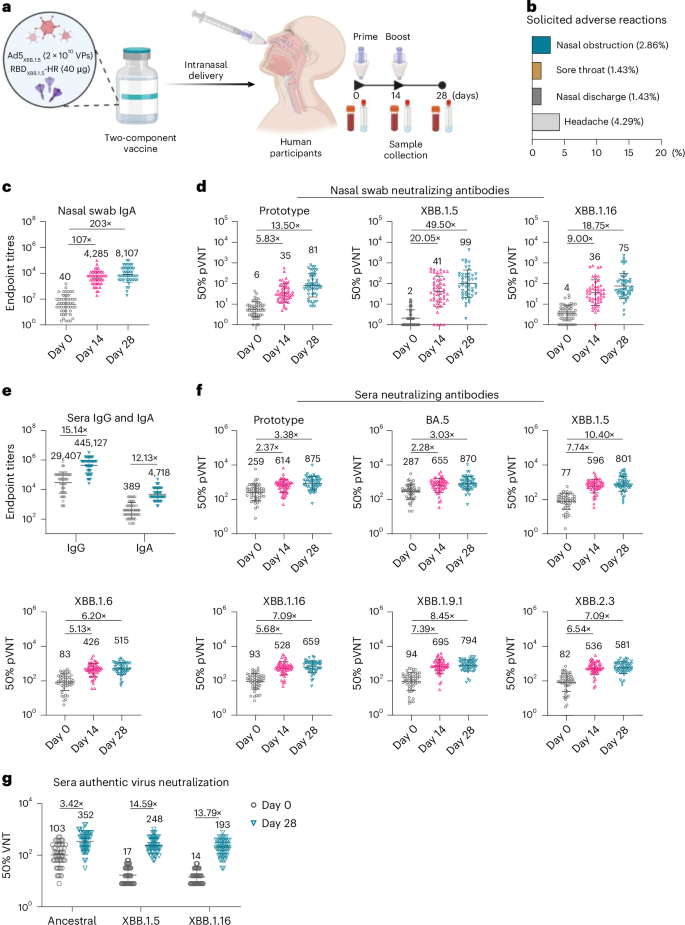
a, Schematic representation of the investigator-initiated trial to evaluate the safety and immunogenicity of the two-component vaccine in humans. Participants were intranasally administered with Ad5XBB.1.5 + RBDXBB.1.5-HR (n = 20 participants received 1 × 1010 VPs of adenovirus plus 20 μg protein and n = 50 participants received 2 × 1010 VPs of adenovirus plus 40 μg protein) on days 0 and 14, and blood and nasal wash samples were collected to detect the levels of binding and neutralization antibodies. b, Common adverse reactions within 14 days of each vaccination in all human participants (n = 70 human participants). c, Endpoint titres of anti-RBDXBB.1.5 IgA in nasal swab samples from participants in the group receiving 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR (n = 50 participants). d, Neutralizing antibodies against prototype, XBB.1.5 and XBB.1.16 pseudoviruses in nasal swab samples in the group receiving 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR (n = 50 participants). e, Endpoint titres of sera IgG and IgA in human participants on day 28 (n = 50). f, Sera neutralizing antibodies against pseudoviruses (prototype, BA.5, XBB.1.5, XBB.1.6, XBB.1.9.1, XBB.1.16 and XBB.2.3) were determined (n = 50 participants). g, Neutralizing antibodies against authentic ancestral, XBB.1.5 and XBB.1.16 viruses in sera collected on day 28 (n = 50 participants). The numbers above the data points in c–g indicate the GMTs of 50% neutralization measured for pre- and post-immunization samples, as well as the fold changes between them. Data are presented as geometric mean ± s.d. Schematic in a created with BioRender.com.
Another objective of this investigation was to assess the mucosal and systemic immune response induced by the intranasal two-component vaccine. Blood and nasal swab wash samples were collected before the first vaccination (used as pre-immunization control) and on days 14 and 28 for immunological assays. We first measured the mucosal binding antibodies in nasal wash samples from 50 participants in the group receiving 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR, and found significant enhancement of antigen (RBDXBB.1.5)-specific IgA levels after intranasal vaccination (Fig. 6c). Compared with pre-immunization levels, the titres of IgA antibody were 107-fold higher after the first intranasal delivery, and further increased by 203-fold after the second vaccination. In addition to the improvement in mucosal IgA, neutralizing activities were also significantly improved by the two-component vaccines (Fig. 6d). The GMTs of neutralizing antibody against prototype, XBB.1.5 and XBB.1.16 pseudoviruses were improved by 5.83-, 20.05- and 9.00-fold, respectively, after the first dose of immunization, and improved by 13.50-, 49.50- and 18.75-fold, respectively, after the second dose.
In the next set of experiments, sera binding and neutralizing antibody responses were examined, and the GMTs for sera binding IgG and IgA reached 445,127 and 4,718, respectively (Fig. 6e). The results of pseudovirus neutralization showed that most participants have detectable sera neutralizing antibodies induced by previous vaccination and infection, while the neutralizing capacities were extensively compromised by XBB lineages. Intranasal delivery of the two-component vaccine rescued the neutralizing activities against all circulating XBB subvariants (Fig. 6f). The plasma neutralizing antibodies were significantly increased at 14 days after the first vaccination, and the GMTs of 50% neutralization against Prototype, BA.5, XBB.1.5, XBB.1.6, XBB.1.16, XBB.1.9.1 and XBB.2.3 were improved by 2.37-, 2.28-, 7.74-, 5.13-, 5.68-, 7.39- and 6.54-fold, respectively. At 28 days after the first vaccination, the GMTs of 50% neutralization against XBB.1.5, XBB.1.6, XBB.1.16, XBB.1.9.1 and XBB.2.3 variants reached 801, 515, 659, 794 and 581, respectively, highlighting strong neutralizing potency against all XBB lineages. Furthermore, serum samples were subjected to authentic virus neutralization assays. The results exhibited similar significant improvements in serum neutralizing activities against pseudoviruses after the second intranasal vaccination, with GMTs against the ancestral, XBB.1.5 and XBB.1.16 viruses reaching 352, 248 and 193, respectively (Fig. 6g). Similar improvements in neutralization were also observed in the group receiving a two-component intranasal vaccine containing 1 × 1010 VPs of Ad5XBB.1.5 plus 20 μg of RBDXBB.1.5-HR protein (Extended Data Fig. 7). These results suggest a good immunogenicity of the nasal two-component vaccine. Therefore, this clinical trial demonstrates the safety and strong immunogenicity of the intranasal two-component vaccine in humans, inducing robust mucosal and systemic immune responses.