Phytosubstances of TCFUV–Vis characteristics
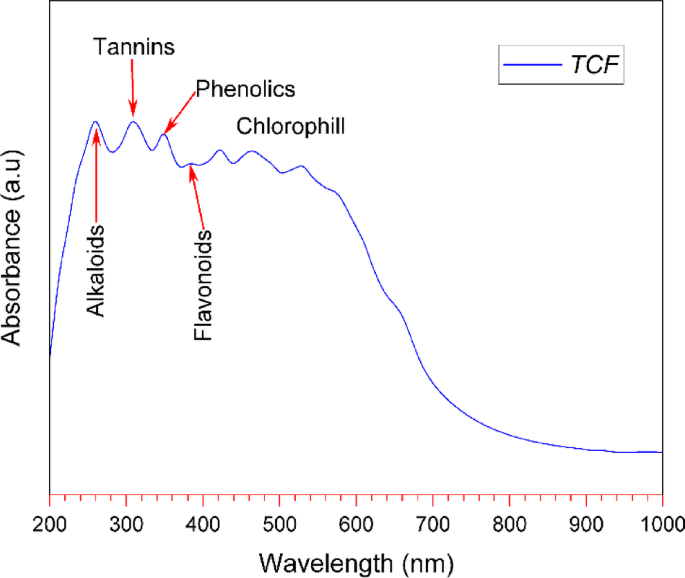
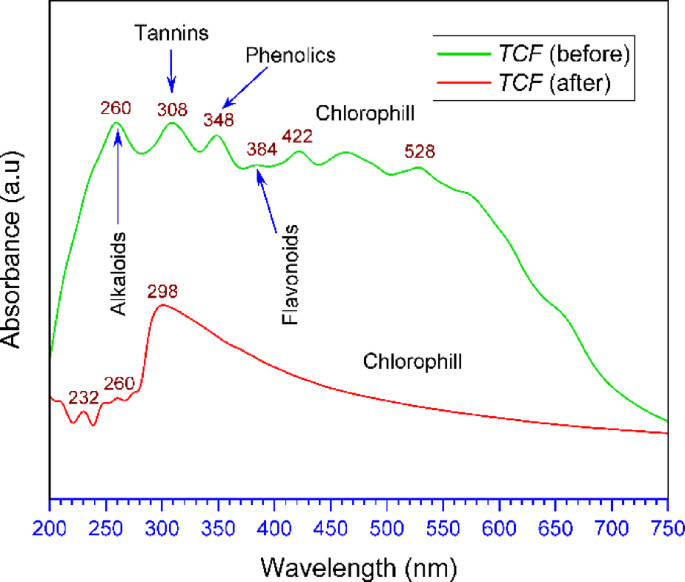
The results of the UV–Vis spectrum of the TCF are shown in Fig. 1. The spectrum of the TCF shows several absorbance peaks in the range of 260–550 nm. The bands at 260 nm and 308 nm indicate the presence of alkaloid and tannin compounds, respectively. Phenolic compounds are seen at the peak of the 348 nm band due to their aromatic hydroxyl groups. Flavonoid compounds are detected at an absorption of around 384 nm due to the presence of a conjugated ring structure. Furthermore, the peak range of 422 nm to 528 nm is considered characteristic of chlorophyll compounds. This chlorophyll absorbance band aligns with the findings reported in the ethanol extraction of Dillenia suffruticosa leaves32.
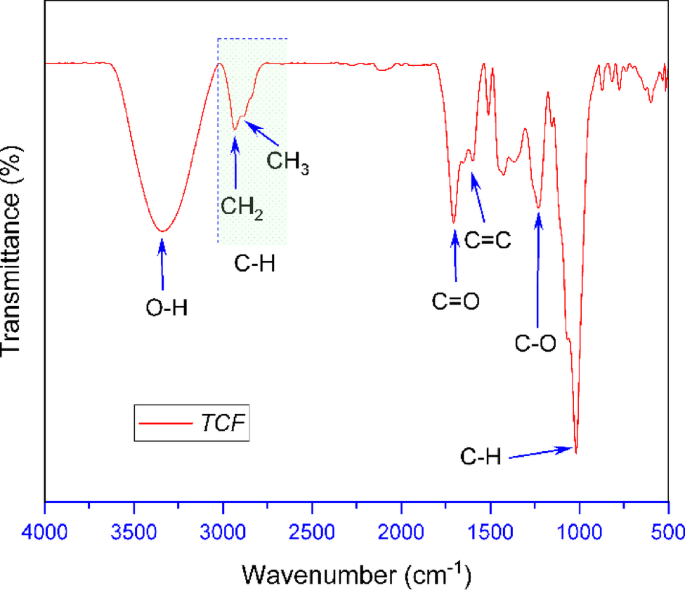
FT-IR (Fourier Transform Infrared Spectroscopy) analysis was employed to identify the functional groups present in compounds contained in the TCF, as well as to elucidate the chemical structure of active compounds that may contribute to the corrosion inhibition of carbon steel in seawater media. The results of the TCF spectra measurements are shown in Fig. 2.
The wide band in the valley (3300–3350 cm1) indicates the presence of hydroxyl groups (O–H groups). This band is a characteristic of polyphenol, flavonoid, or tannin compounds that have the potential to be corrosion inhibitors by forming a protective layer on the surface of carbon steel. The C–H group was identified at 2800–3000 cm1, and this band indicates the presence of alkyl compounds or compounds with alkyl groups in the structure. In addition, a carbonyl group (C=O) at 1704 cm−1 was also detected, which can interact with the surface of carbon steel through the adsorption process. In TCF, double bonds in aromatic compounds, such as flavonoids or polyphenols, were also detected in the band 1600 cm−1. Furthermore, in the band 1225 cm−1 (C–O group), it is suspected that the compound is an ester or phenolic compound, essential in increasing the metal surface’s affinity and corrosion inhibition efficiency.
FT-IR analysis revealed that the TCF contains bioactive compounds, including phenols, flavonoids, and esters, which possess active functional groups such as –OH, C=O, and C=C, potentially playing a role in inhibiting the corrosion process in carbon steel. These compounds can potentially adsorb on the surface of carbon steel, forming a protective layer that reduces the interaction of the metal with corrosive media. Therefore, the active compounds in Tinospora cordifolia can effectively protect against corrosion, especially in seawater environments.
HR-MS analysis
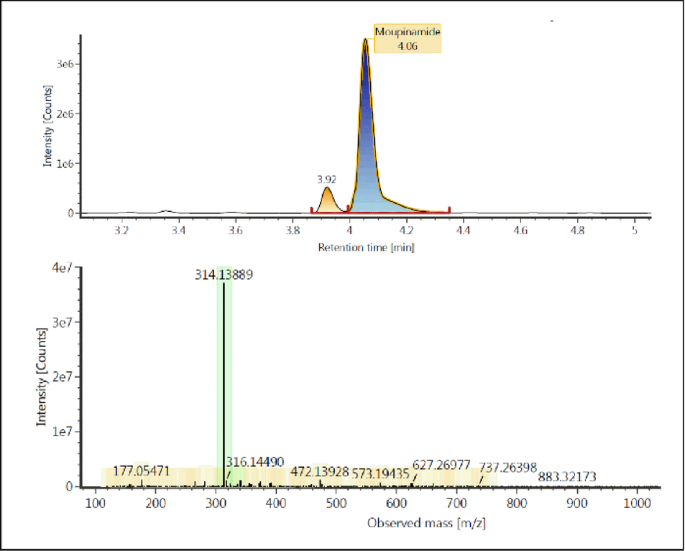
HR-MS (High-Resolution Mass Spectrometry) analysis was employed to determine the molecular weight and structure of compounds present in the TCF, as well as to identify bioactive compounds with the potential to act as corrosion inhibitors on carbon steel in seawater media. The results of the TCF spectra with HR-MS are shown in Fig. 3.
The spectral results in Fig. 3 show several peaks, with the prominent peak detected at m/z (mass-to-charge ratio) 314. This peak indicates a possible compound with a molecular weight of around 313 Da. Based on literature data, this may be related to flavonoids or phenolics, often found in Tinospora cordifolia33. There is also a peak at m/z 177, indicating a compound with a smaller structure, most likely a phenolic derivative or a flavonoid compound, which also contributes to the inhibition mechanism.
NMR analysis
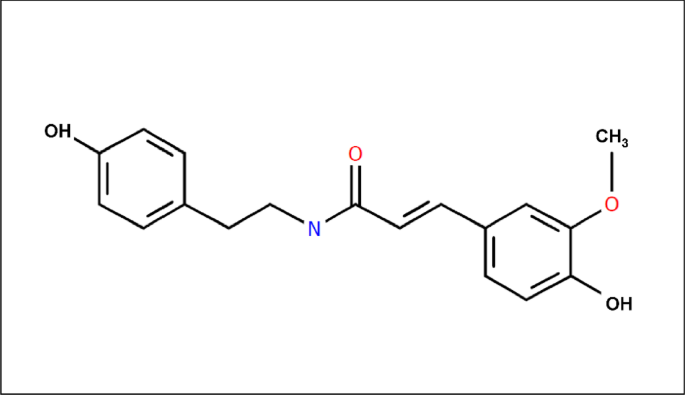
The NMR results on the dominant compound of the TCF are shown in Fig. 4. Based on the results of specific H-NMR, C-NMR, and 2D-NMR spectra, this compound is suspected to be N-(4-Hydroxy-3-methoxy-E-cinnamoyl)-4-(2-aminoethyl)phenol, also known as 4-(2-aminoethyl) N-trans-feruloyltyramine and Moupinamide. This finding is reinforced by the HRMS results, where the compound with a molecular weight of 313 (moupinamide) is the dominant compound in the TCF.
NMR spectra of the compound from TCF.
Moupinamide can act as an inhibitory agent for corrosion activity on carbon steel in seawater media, considering that amide compounds often interact with metal surfaces34. The amide group (–CONH–) in Moupinamide can interact with metal surfaces through physical or chemical adsorption, forming a protective layer that reduces the interaction between metal and corrosive media. In addition, these compounds, especially those with amide and aromatic groups, can act as antioxidants, reducing oxidation reactions that occur on carbon steel in seawater media35.
Electrochemical studyOpen circuit potential (OCP) analysis
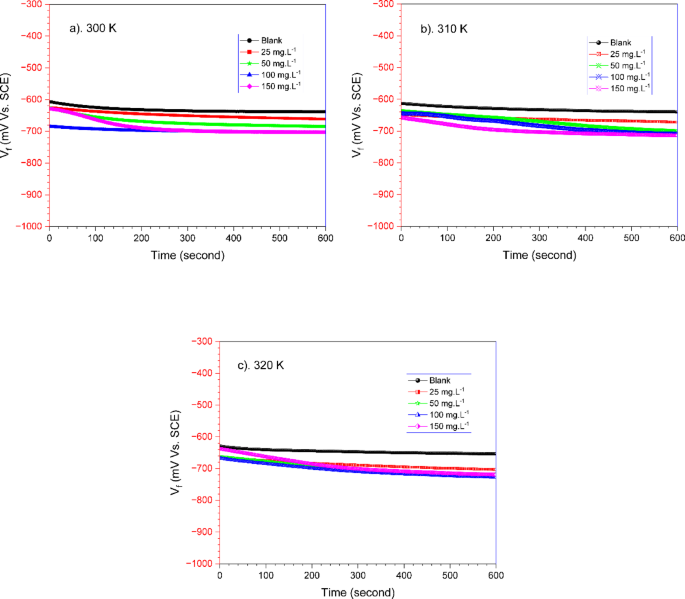
Open-circuit potential (OCP) measurements were performed to determine potential stability before applying the current. The results of the OCP graph for 600 s on carbon steel with and without TCF at various temperatures are presented in Fig. 5. The shift in the OCP value shifted negatively, indicating ongoing corrosion. However, in the presence of TCF, the OCP value stabilized over time, indicating the formation of a protective inhibitor layer on the steel surface. The stabilisation of the OCP over time further confirms the formation of a durable inhibitor layer that minimises the anodic and cathodic reactions responsible for corrosion. A limited range of potential shifts is − 606.9 mV to − 703.7 mV, − 612.9 mV to − 713.3 mV, and − 629.2 mV to − 727.7 mV for 300 K, 310 K, and 320 K, respectively. This limit of OCP may reflect the type of mixed inhibitors with cathode dominance36.
Open circuit potential (OCP) curves of carbon steel in seawater with the absence and presence of varying TCF at (a) 300 K, (b) 310 K, and (c) 320 K.
On the other hand, it has been suggested that the shift towards more negative corrosion potentials is related to the absorption of inhibitors or desorption of corrosion products from the metal surface37. In addition, increasing temperature also causes a shift in the OCP value to be increasingly negative38. Similar behaviour also occurs in aloe saponaria extract39, where the OCP moves negatively with the addition of extract.
Potentiodynamic polarization (PDP) analysis
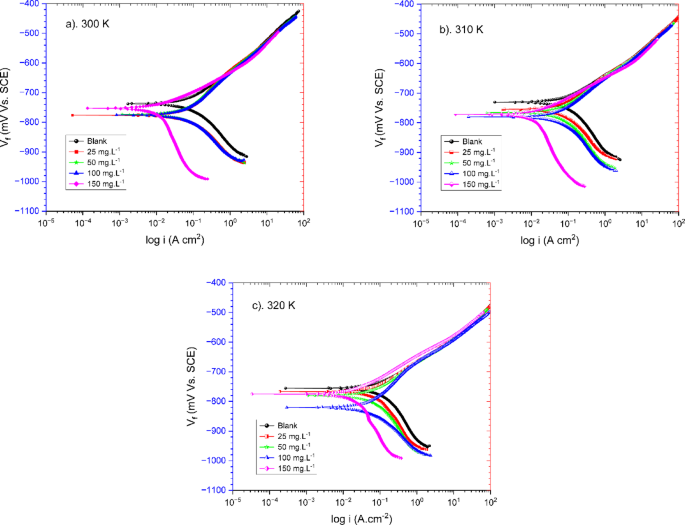
The potentiodynamic polarization (PDP) measurements were conducted on carbon steel samples in a seawater medium with varying concentrations of TCF as an organic inhibitor, ranging from 25 to 150 mg.L−1, at different temperatures. Polarization curves were recorded (Fig. 6), and key parameters involving corrosion current density (Icorr), Tafel slopes (βc), and corrosion potential (Ecorr) were extracted from the polarization plots as tabulated in Table 2. The PDP curve results show that the corrosion potential (Ecorr) shifts toward negative values as the concentration of TCF increases. This decrease in corrosion potential indicates a tendency for the carbon steel surface to react with increasing concentrations of TCF40. The corrosion potential (Ecorr) also shifts negatively with increasing temperature. This negative shift indicates that the activation energy for the corrosion process decreases, making the process more thermodynamically favorable and effortless to initiate at higher temperatures41.
Potentiodynamic polarization (PDP) curves of carbon steel in seawater with the absence and presence of varying TCF at (a) 300 K, (b) 310 K, and (c) 320 K.
Table 2 Potentiodynamic electrochemical parameters of carbon steel in seawater containing TCF at different temperatures.
The cathodic slope (βc) results in Fig. 6 provides information about the rate of oxygen reduction, which in this work tends to be cathodic inhibition. The βc value changes with TCF, indicating a change in the cathode hydrogen evolution mechanism. This case suggests that the active substances in TCF significantly slow down carbon steel corrosion as their inhibition capacity increases with concentration. The βc value changed slightly from 25 mg.L-1 to 100 mg.L− 1, indicating a minimal effect on the cathodic process. However, at a concentration of 150 mg.L− 1, βc tended to increase, indicating that the inhibitor inhibited oxygen reduction, which is essential in reducing the corrosion rate42. The relatively unchanged Tafel slope (although slightly cathodic) suggests that the TCF does not alter the fundamental corrosion mechanism but instead reduces the overall rate of the anode and cathode processes43. This type of inhibition may be due to the chemical structure of the TCF molecule, which contains functional groups that can interact with metal surface atoms and reduce oxygen in solution42. From the polarization curve in Fig. 6 and the data in Table 2, it was also identified that the Tafel beta slope (βc) changed with increasing temperature. Under conditions with the addition of TCF, it tended to be smaller (less sharp) than in the blank solution. This shift in the Tafel slope confirms that the TCF affects the corrosion process (cathode) across all temperature ranges, a characteristic of the inhibitor44.
The PDP curve (Fig. 6) also shows a decrease in extensive corrosion current density (Icorr) with TCF. The reduction in corrosion current density (Icorr) and the negative change in corrosion potential (Ecorr) indicate that TCF molecules are adsorbed onto the steel surface45, thereby establishing a protective film46. This film effectively blocks the active sites, inhibiting the electrochemical reactions responsible for metal dissolution46. The inhibition efficiency is moderate at low concentrations of TCF (25 to 100 mg.L−1 TCF). The drop in corrosion current density (Icorr) is apparent under these conditions, implying that the inhibitor may function by adsorbing onto the steel surface and forming a protective layer. However, the effect is less pronounced than at higher concentrations (150 mg.L−1). This result implies that < 100 mg.L−1 TCF can provide some protection but may not fully prevent corrosion in this condition. Furthermore, at higher concentrations (150 mg.L−1), Icorr decreased significantly, indicating that the inhibitor provided stronger protection, with a clear decrease in the corrosion rate.
However, the corrosion current density (Icorr) increases with temperature due to the increasing rate of iron anode dissolution and hydrogen ion cathode reduction (H⁺)47. At 300 K, Icorr shows a lower value with a stronger inhibitor effect. At 310 K, Icorr increases slightly, but the inhibitor effect is still visible. At 320 K, Icorr increases sharply, indicating that high temperatures reduce the effectiveness of the inhibitor and increase the corrosion rate. In conditions without an inhibitor, Icorr is approximately 102.70 µA/cm2 at 300 K and increases to 113.80 µA/cm2 at 320 K. This increase in corrosion current indicates that temperature accelerates the corrosion process. As a result, the corrosion rate increases significantly at high temperatures. Under conditions with TCF (150 mg.L−1), Icorr was 9.36 µA.cm−2 at 300 K and increased to 18.50 µA.cm−2 at 320 K. Although there was still an increase in Icorr with temperature, the increase was significantly smaller than that of the Icorr in the blank specimen, reflecting the inhibitor’s ability to reduce the corrosion rate. This case demonstrates that the TCF inhibitor effectively inhibits the corrosion process, even at high temperatures, although its effectiveness (IE) decreases slightly with increasing temperature.
The inhibition efficiency of TCF was determined using the approach described by Eq. (1), which resulted in inhibition efficiency (%IE) values increasing with increasing TCF concentration. The increase in inhibition efficiency with TCF concentration up to a certain point is consistent with the typical behaviour of corrosion inhibitors. A relatively low IE was generated at low concentrations (below 100 mg.L−1), possibly due to insufficient adsorption to completely cover the metal surface, leading to a lower inhibition rate (66.23% for 100 mg.L−1). As the concentration increased (150 mg.L−1), more inhibitor molecules were available to adsorb on the steel surface, leading to a higher inhibition efficiency (about 90.89%) at 300 K. At this concentration of TCF, the inhibition efficiency reached its highest value, indicating that this concentration is optimal for reducing the corrosion rate of carbon steel in seawater media well.
Potentiodynamic polarisation data measurements (Table 2) confirmed that TCF acted as an effective corrosion inhibitor for carbon steel in a seawater medium. The declining trend in the corrosion current density at higher concentrations indicated that TCF could be preferentially adsorbed to the active regions on the steel surface, thereby creating a barrier to corrosive species48. The inhibition efficiency of TCF observed in this study is comparable to that of other organic inhibitors, such as rice straw extract (92%) in a NaCl solution49. Additionally, this result aligns with previous studies on the effect of inhibitor concentration on seawater media, where efficiency increases with increasing concentration50. These efficiency results emphasize the promise of TCF as a corrosion inhibitor, although further investigation is recommended to explore its long-term stability and effectiveness in real-world applications.
Electrochemical impedance spectroscopy (EIS) analysis
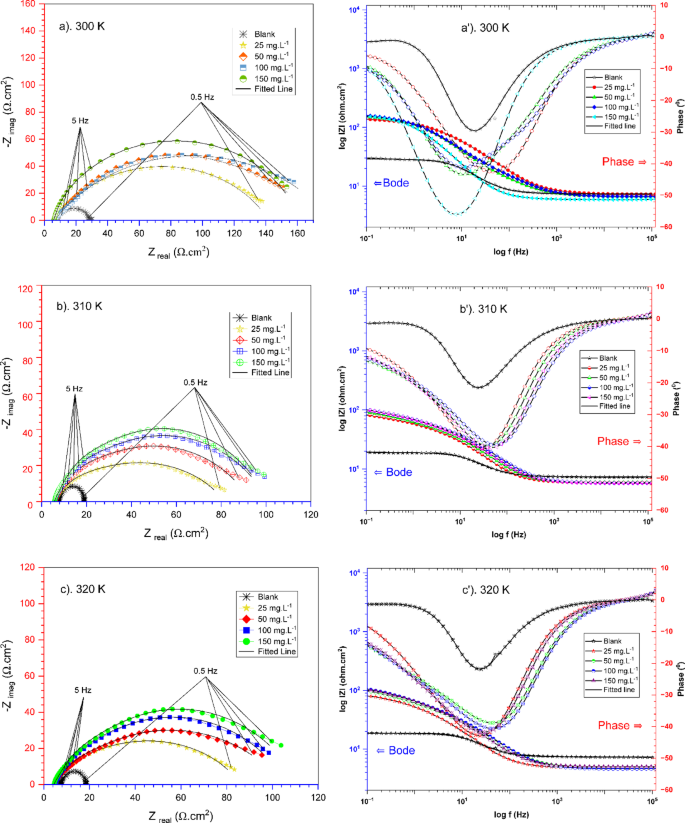
Figure 7 displays the effects of different TCF concentrations (blank, 25 mg.L−1 to 15 mg.L−1) on the performance of carbon steel using an electrochemical technique in a seawater medium. The electrochemical parameter data from fitting using a circuit model (in Fig. 8) were tabulated in Table 3.
Nyquist and Bode plots of carbon steel in seawater with the absence and presence of varying TCF at (a and a’) 300 K, (b and b’) 310 K, and (c and c’) 320 K.
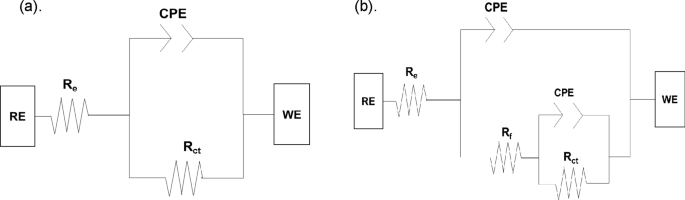
The circuit model used in interpreting TCF performance analysis is (a) blank and (b) with TCF.
Table 3 Electrochemical parameters for carbon steel in seawater with different temperatures and TCF concentrations.
As shown in Figs. 7a, b, c, the Nyquist plot results display a semicircular curve associated with charge transfer resistance (Rct) and double-layer capacitance (Cdl). According to the Nyquist plot, the diameter of the semicircle increases as TCF concentration increases. This results in a rise of charge transfer resistance (Rct), which implies a reduction in corrosion rate11. The addition of TCF enhances the formation of a protective layer on the carbon steel surface, thereby protecting against the electrochemical reactions that cause corrosion51. At the lowest concentration of TCF (25 mg.L−1), the resulting Rct is relatively low, indicating limited protection. The increase in corrosion resistance occurs at concentrations of 100 mg.L−1 and 150 mg.L−1, suggesting that forming a perfect passive layer more effectively inhibits corrosion52. Higher Rct values indicate more efficient corrosion inhibition, as the TCF molecules interact with the steel surface, reducing the active area accessible for corrosive attack51. Furthermore, increasing temperature causes a decrease in Rct, indicating that the temperature effect can reduce the inhibitor’s ability to form an effective passive layer53.
The Bode plot results (Figs. 7a’, b’, c’) confirm the stability of the protective layer, where at a concentration of 25 mg.L−1, it shows a lower impedance at low frequencies, indicating a less stable passive layer. At concentrations of 100 mg.L−1 and 150 mg.L−1, it exhibits a very high impedance, indicating the formation of a stable and effective protective layer. This result is also reinforced by the phase angle value, which increases with the addition of inhibitors, indicating that the capacitive properties of the protective layer become increasingly stable and uniform54. The Bode plot and phase angle show a decrease with increasing temperature, indicating a reduction in the stability of the protective layer51. The Nyquist and Bode plots in this study suggest that TCF is an effective inhibitor of carbon steel in seawater. The increments in charge transfer resistance (Rct) and the improvement in phase angle (θ) at higher TCF concentrations indicate the potential to establish a stable, protective barrier on the steel surface. These electrochemical results are consistent with the previous study46, which has demonstrated the effectiveness of ascorbic acid as a green corrosion inhibitor for API 5L × 60 by adsorption on the metal surface and creating a protective barrier against aggressive ions in seawater environments.
Table 3 summarises the EIS parameter data by matching the spectrum data to the corresponding electrical model illustrated in Fig. 8, which provides a better understanding of the electrochemical behaviour. The components of this electrical circuit include electrolyte resistance (Re), charge transfer resistance (Rct), film resistance (Rf), and a constant phase element (CPE). Because an inhomogeneous steel surface is considered, the double-layer capacitance (Cdl) was replaced by the constant phase element (CPE) to provide a more accurate matching38. The CPE impedance function is represented in the following Eq. 1155:
$$Z_{CPE} = Y_{0}^{ – 1} (i\omega )^{ – n}$$
(11)
In this case, the notation of i,ω, Y0, and n represents the imaginary unit (i2 = − 1), angular frequency (ω = 2πf), the CPE constant, and the CPE exponent, respectively, with values spanning from − 1 to 1. Additionally, the subsequent formula (Eq. 12) establishes a relationship between the CPE and Cdl56:
$$C_{{{\text{dl}}}} = \sqrt[n]{{Y_{0} .R_{P}^{1 – n} }}$$
(12)
The data in Table 3 reveal that the electrolyte resistance (Re) tends to decrease at higher temperatures. At higher temperatures, the molecules in the solution become more active, which can increase the corrosion rate41. This increase is attributed to the acceleration of the oxidation process in the metal, resulting in a decrease in electrolyte resistance (Re)29. At higher temperatures, although the inhibitor still functions, the increase in the corrosion rate can reduce the effectiveness of the inhibitor protection, leading to a decrease in resistance. The decreasing trend of n values with the concentration amount is due to the increasing non-uniformity of the substrate produced by the active molecules adsorbed on the steel surface57,58. The rising trend of Rct values with increasing concentrations of TCF indicates that the active molecules of TCF are adsorbed on the carbon steel surface exposed to seawater media, blocking the active areas exposed to corrosive agents and producing a thin protective layer at the metal-solution interface.
The results showed that temperature has a substantial impact on the corrosion reaction of carbon steel in seawater media. In the absence of TCF, the corrosion rate increased significantly with temperature, as expected, due to the improved reaction kinetics51. The addition of TCF reduced this temperature-dependent corrosion acceleration, making the effect more significant as the inhibitor concentration increased. With 150 mg.L−1 of TCF, the inhibition efficiency was high throughout the temperature range, although it decreased slightly with increasing temperature. At 300 K, the efficiency (%η) was about 92.25%, while at 310 K, the efficiency (%η) decreased to about 90.14%. This decrease is likely due to the reduced efficiency of inhibitor adsorption at higher temperatures59,60, which can weaken the protective layer. However, at 320 K, despite the decreased efficiency, the inhibitor still provided significant protection, reducing the corrosion rate by about 89.69% compared to the blank specimen. The decrease in inhibition efficiency at high temperatures is due to the decreased adsorption strength of the TCF on the steel surface. At high temperatures, the molecular reaction process between the steel surface and the inhibitor becomes weaker61,62, which causes a decrease in the effectiveness of the protective layer.
Thermodynamic and adsorption studies
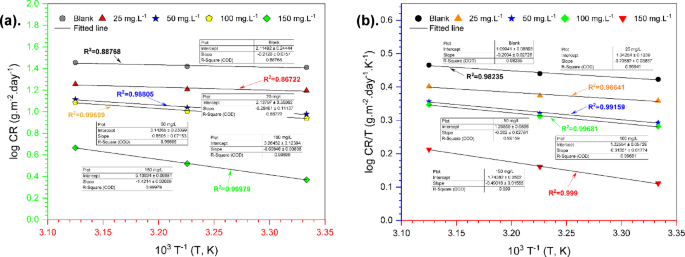
To fully understand the effectiveness of TCF as a corrosion inhibitor, it is important to investigate its behavior through thermodynamic, kinetic, and adsorption analysis. These analyses help determine the inhibition mechanism, activation energy, adsorption characteristics, and the correlation between temperature and inhibitor concentration. Increasing temperature can significantly influence the characteristics of steel materials, including kinetics, corrosion rate, and equilibrium. The Arrhenius equation, expressed in Eq. (13) 63, can be applied to investigate the relationship between corrosion rate and temperature factors.
$${\text{log CR}} = \frac{{ – E_{a} }}{2.303R}\left( \frac{1}{T} \right) + {\text{log }}A$$
(13)
$${\text{log }}\frac{CR}{T} = \left( {{\text{log}} \frac{R}{{N_{A} h}} + \frac{{\Delta S_{a} }}{2.303R}} \right) – \left( {\frac{{\Delta H_{a} }}{2.303R}\frac{1}{T}} \right)$$
(14)
The notation \({E}_{\text{a}}\), A, R, \(\Delta {H}_{\text{a}}\), \(\Delta {S}_{\text{a}}\), h, NA, and T represent the activation energy, the Arrhenius factor, the gas constant, the activation enthalpy, the activation entropy, the Planck constant, the Avogadro number, and the temperature in kelvin, respectively. Figure 9a shows how the slop of the log (CR) = f (103 T−1) curve was applied to determine \({E}_{\text{a}}\). Table 4 lists the values of the Arrhenius factor (A), \(\Delta {S}_{\text{a}}\), and \(\Delta {H}_{\text{a}}\), which were obtained from both the intercept and the slope of the log (CR/T) = f (103 T−1) curve (Fig. 9b) followed in Eq. (14) 64.
Arrhenius plots (a) and Arrhenius transition plots (b) for carbon steel in seawater medium in the absence and presence of the inhibitor TCF.
Table 4 Thermodynamic activation parameters for carbon steel resulted in the absence and presence of various TCF concentrations.
The corrosion current density (Icorr) exhibits an Arrhenius-type behaviour with temperature, indicating that the corrosion rate increases with rising temperature. The activation energy (Ea) for corrosion can be determined by plotting ln (Icorr) vs. 1/T. For the blank solution, the activation energy (Ea) obtained was 4.07 kJ mol−1 (lower than the condition with the addition of TCF), indicating that the corrosion mechanism is thermodynamically favourable and easy to initiate. The increase in Ea (5.45 to 27.22 kJ.mol−1) suggests that the inhibitor effectively blocks the corrosion reaction65, which requires more energy to initiate the electrochemical reaction. This behaviour is typical of inhibitors adsorbed on the metal surface, slowing down the corrosion reactions66. Several studies have shown that Ea for the blank solution tends to decrease with increasing temperature, indicating that corrosion becomes easier to initiate at higher temperatures29. On the contrary, the activation energy slightly increases with the presence of an inhibitor, indicating that the inhibitor reduces the thermodynamic properties of corrosion and makes it difficult for the electrochemical reaction to occur, even at higher temperatures27. The positive value of ΔH illustrates the endothermic process in the reaction67.
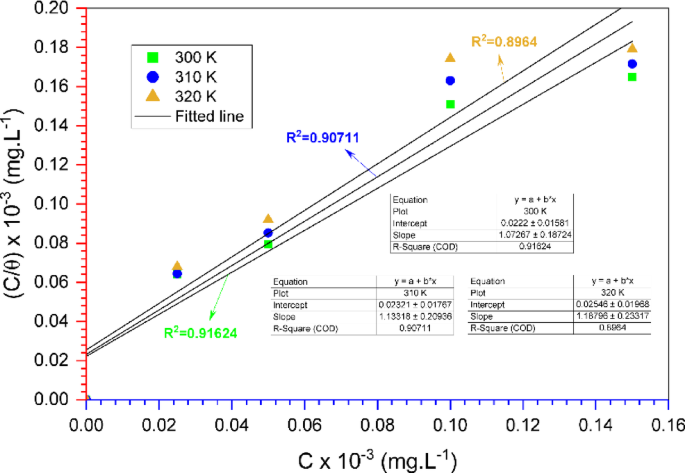
Thermodynamic parameters of corrosion inhibition, such as adsorption-free energy (ΔGads), are essential to understanding the effectiveness of inhibitors. The Langmuir adsorption isotherm was employed to assess the adsorption of TCF onto steel surfaces. Langmuir isotherm interprets that the adsorption sites on the steel surface are equivalent and that the adsorption of inhibitor molecules occurs independently, without interaction between the adsorbed molecules. The adsorption isotherm can be formulated as follows in Eq. (15) 68:
$$\frac{C}{\theta } = \frac{1}{K} + C$$
(15)
where θ denotes the surface fraction covered by the inhibitor (IE/100), C represents the inhibitor concentration, and Kads is the adsorption equilibrium constant.
From the Langmuir plot (C/θ vs. C) as displayed in Fig. 10, a linear relationship can be obtained, and the slope of the line represents 1/Kads, which allows the determination of the adsorption constant. The higher the Kads, the stronger the correlation between the steel surface and the inhibitor, indicating a better inhibition effectiveness.
Langmuir adsorption isotherm of TCF on carbon steel in seawater medium.
Furthermore, the standard adsorption free energy (ΔGads) is the main parameter that could be calculated from the adsorption equilibrium constant (Kads) using the following Eq. (16) 51:
$$\Delta G_{ads} = – 2.303RT\log \left( {10^{6} \cdot K_{ads} } \right)$$
(16)
In this adsorption case in the corrosion field, R and 106 denote the gas constant and H2O concentration, respectively. Table 5 depicts the adsorption parameters of TCF on carbon steel surfaces in seawater. In conditions without an inhibitor (blank), there is no adsorption process; therefore, ΔGads is not valid (not determined). In the presence of TCF, ΔGads is obtained around − 26.73 kJ.mol−1 to − 28.15 kJ.mol−1, a negative value characteristic of the spontaneous adsorption5. Various literatures show that chemisorption occurs when the ΔG value is less than − 40 kJ/mol. The combined physisorption-chemisorption process, or mixed type, occurs when the ∆G value is between − 40 and − 20 kJ.mol−1. Furthermore, physisorption occurs when the ΔG value exceeds − 20 kJ/mol. In this work, the ΔGads value, ranging from − 26.73 kJ/mol to − 28.15 kJ/mol, indicates that the inhibitor exhibits a mixed type of physisorption-chemisorption adsorption onto the steel surface69, resulting in a correlation between the carbon steel surface and the inhibitor.
Table 5 Adsorption parameters for the concentration effect of carbon steel in seawater medium at 300 K, 310 K, and 320 K.
The inhibition mechanism of TCF is likely to inhibit corrosion through physical and chemical adsorption mechanisms, forming a protective barrier on the steel surface. The efficiency of the inhibitor rises with concentration, although its effectiveness decreases with increasing temperature. The ability of the inhibitor to decline the anodic dissolution process (Fe → Fe2⁺) and cathodic hydrogen evolution (2H⁺ + 2e⁻ → H₂) indicates a mixed-type inhibition mechanism. At lower concentrations, TCF is predicted to be adsorbed to the steel surface through van der Waals forces or electrostatic interactions, forming a protective film that prevents direct contact between the metal surface and the corrosive medium. The effectiveness of physical adsorption is temperature dependent, with higher temperatures potentially weakening the adsorption bond70. At higher concentrations or temperatures, TCF can form stronger bonds with the steel surface, indicating chemisorption69. The presence of nitrogen and amines in the TCF structure can facilitate the formation of coordinate bonds with the steel surface, thereby enhancing the protective effect of the inhibitor.
Comparison, potential application, and future studies
Detailed comparisons with other natural extracts, insights into applications, and suggestions for future studies are crucial for highlighting the significance of the findings and exploring their broader implications. Numerous studies have investigated the use of natural plant extracts as corrosion inhibitors for carbon steel. For example, Carob fruit, Bidens pilosa, Manihot esculenta, Catharanthus roseus, and Tinospora crispa have been well documented. These studies reported corrosion inhibition efficiencies in the 60–90% range in almost the same media, namely sodium chloride or seawater environments, as tabulated in Table 6. In terms of inhibition efficiency, the efficiency in this study is more competitive than that of several previous studies, where remarkable corrosion inhibition efficiencies were achieved (90.89% with PDP and 92.25% with EIS) at a relatively low dose (150 mg.L−1). These higher efficiency results are possible because the active compounds are purer than in previous studies27, where the extracts were initially fractionated, allowing the role of the active substances to be more optimal than that of the crude extract. However, some natural extracts, such as Nigella sativa L. and Apple pomace, showed slightly higher efficiencies, with 94% and 98.8% efficiency values, respectively9,71. The increase in inhibition efficiency can be attributed to the increased concentration applied and decreases with increasing temperature40,41. Different inhibition efficiencies are also influenced by the various types of extract solvents used because they produce different bioactive compounds72.
Table 6 Comparative efficiency of TCF with several previous studies.
Furthermore, the presence of functional groups such as –OH, –NH₂, –C=O, and aromatic rings can enhance adsorption on metal surfaces by donating electrons or forming coordinate bonds with the metal surface73. In addition to the preparation of extracts and the concentration of inhibitors, the type and concentration of corrosive species, such as chloride (Cl−) and sulfate (SO₄2 −), as well as aggressive ions, also influence the effectiveness of inhibitors because they can form complexes with corrosive ions, thereby reducing their effectiveness74. Furthermore, electron-donating capacity (HOMO–LUMO gap) and polarizability also contribute to differences in inhibition ability. Therefore, quantum chemical computations are crucial for predicting and rationalizing inhibition performance.
The potential utilization of Tinospora cordifolia extract from this study presents a more environmentally friendly and sustainable alternative to traditional synthetic inhibitors, which may be toxic or harmful to the environment. The practical application of TCF can be applied to structures in contact with seawater where corrosion resistance in saline environments is critical. In addition, TCF can be incorporated into coatings or surface treatments to enhance the corrosion resistance of metals exposed to seawater. Future developments should optimize the extraction method to maximize the concentration of active compounds in Tinospora cordifolia, which are responsible for corrosion inhibition. Standardization of the extract formulation for large-scale applications may make it more feasible for industrial use. Furthermore, long-term studies are necessary to evaluate the durability of its protective effects, particularly under fluctuating seawater conditions and varying temperatures.
Surface morphology studiesSEM–EDX analysis
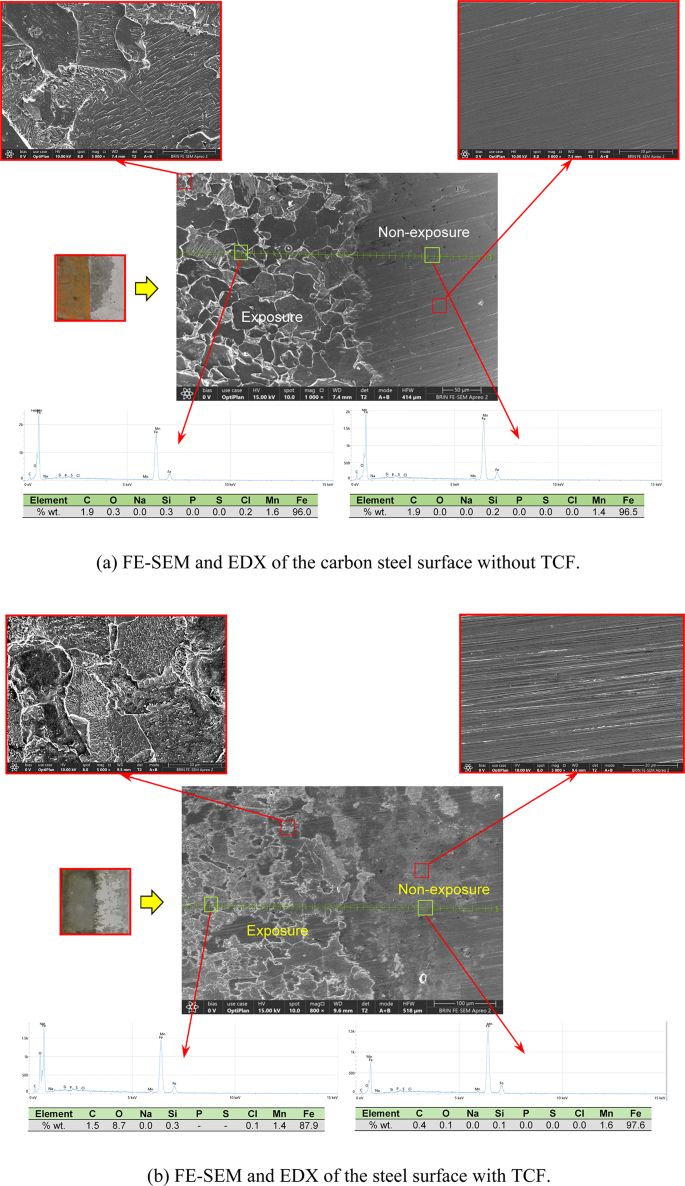
The effectiveness of TCF as a green corrosion inhibitor for carbon steel in seawater medium can be further explained through morphological and surface analysis. Understanding the surface characteristics of carbon steel before and after exposure to inhibitors provides insight into the protection mechanism and effectiveness of bioactive compounds. Figure 11 illustrates the morphological examination of polished carbon steel surfaces before and after being soaked in seawater with the absence and presence of 150 mg.L−1 TCF for 24 h. The carbon steel surface (Fig. 11a) appears well-polished, smooth, and free of spots.
FE-SEM and EDX of the steel surface for (a) without and (b) with TCF.
SEM morphology (Fig. 11b) of carbon steel exposed to seawater media without inhibitors shows significant corrosion features, including peeling, surface roughness, and formation of corrosion products. Corrosion sites indicate an aggressive attack, such as the presence of chloride ions, which cause rapid metal dissolution83. In contrast, SEM images of carbon steel exposed to TCF show a smoother surface with fewer corrosion features. The protective layer formed by the adsorption of bioactive compounds appears to inhibit the penetration of corrosive agents, thereby reducing peeling and surface degradation38. This result suggests that the inhibitor effectively protects mild steel from corrosion. Similar results were found on the surface of AISI 1015 steel substrates exposed to 3.5% NaCl media, where the metal substrate surface displayed severe corrosion in the absence of the green inhibitor (Cassava leaf), and the corrosion decreased significantly with the addition of the inhibitor43. Furthermore, elemental EDX results of the steel surface also show the distribution of elements such as iron, oxygen, and elements associated with Tinospora cordifolia extracts (e.g., carbon from organic compounds). The presence of carbon on the surface after treatment with inhibitors indicates successful adsorption of plant compounds76.
AFM analysis
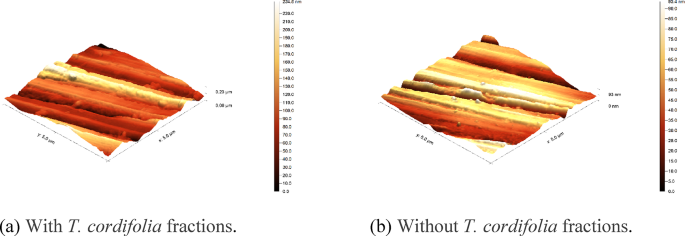
Furthermore, AFM provided high-resolution surface topography images, enabling the assessment of surface roughness and texture. From the AFM results (Fig. 12), the carbon steel sample’s average surface roughness (Ra) value after being added to TCF was much lower than that of the carbon steel after exposure without TCF. The significant reduction in Ra with inhibitors indicates that the surface becomes smoother due to the formation of a protective layer9. The AFM image also shows a homogeneous distribution of the protective layer, confirming the adsorption of active substance molecules from TCF onto the steel surface. The ability of bioactive compounds to cover the surface evenly increases corrosion resistance40.
AFM images of the steel surface for (a) with and (b) without TCF.
UV–Vis analysis
The process of inhibiting steel corrosion in corrosive media with green inhibitors is typically associated with the absorption of active compounds from the extract onto the steel surface. To demonstrate absorption, a UV–Vis test was conducted on the TCF solution before and after exposure, and the results are presented in Fig. 13. The UV–Vis spectrum of TCF before exposure to carbon steel in seawater will show a clear absorption peak (green line), indicating the presence of certain phytochemical compounds, such as alkaloids, tannins, flavonoids, phenolics, or flavonoids, as in section “UV–Vis characteristics“. These compounds have the potential to inhibit corrosion by forming a protective layer on the metal surface. The UV–Vis spectrum shows significant changes after exposure to carbon steel in seawater media (red line). In the spectrum after exposure, there is a shift in the absorption peak (308 nm to 298 nm, Tannin), a reduction in peak intensity for alkaloids, and the disappearance of peaks (phenolics and flavonoids), which will indicate that interactions have occurred between compounds from TCF and the metal surface. The decrease and disappearance of the spectrum in the UV–Vis results of the solution after exposure can be correlated with the corrosion rate data obtained from electrochemical measurements (PDP and EIS), where the inhibition efficiency increases, indicating that phytochemicals play an active role in corrosion inhibition by adsorbing to the metal surface and forming a protective barrier.
UV–Vis spectra of TCF before and after exposure to carbon steel in seawater medium.
XPS analysis
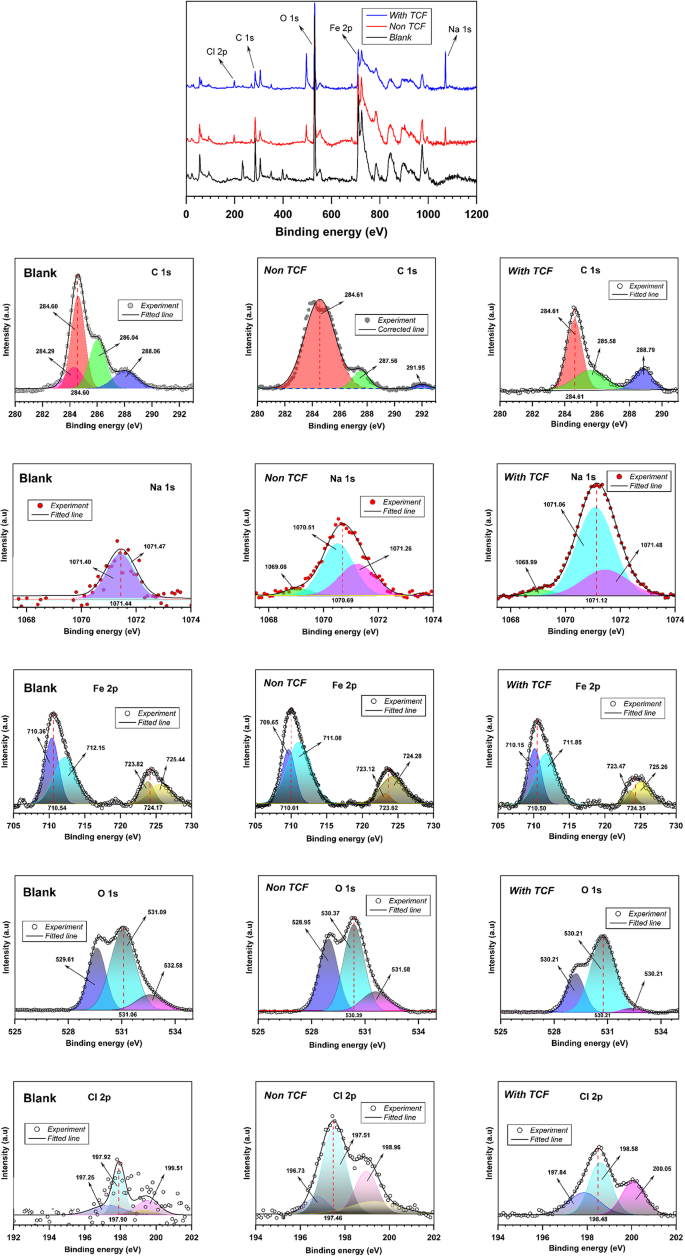
Furthermore, to confirm the chemical composition and state of the elements on the carbon steel surface, an evaluation was conducted using X-ray photoelectron spectroscopy (XPS), with the results presented in Fig. 14. Figure 14 illustrates the XPS survey scan and deconvoluted peaks of each spectrum for the carbon steel surface (blank), exposed steel without and with 150 mg.L−1 TCF. The binding energy values were corrected using the Kratos standard for the signal C1s atom (284.6 eV). The binding energy was calculated using a Shirley-type background and a Gaussian curve. Through the analysis of the electron binding energy in carbon (C 1s), oxygen (O 1s), iron (Fe 2p), and chloride (Cl 2p), valuable data can be obtained, as presented in Table 7. The Na 1 s and Cl 2p peaks were identified on the carbon steel specimen immersed in the solution, indicating that the solution used was rich in Na and Cl elements (seawater).
XPS of carbon steel surface for: blank (left), without TCF (center), and with TCF (right).
Table 7 Element composition and binding energy (BE) for blank, non-TFC, and with TCF of carbon steel by XPS survey.
The C 1 s profile (non-TCF and TCF) showed several peaks at 284.61, 287.56, and 291.95 eV may be labeled as C–C, C=O, and C–O, while in the specimen with TCF at 284.61 eV may be attributed to C–C bonds, 285.58 eV can be recognized as C–O, or C–N bonds, and 288.79 eV can be attributed to N=C bond84. The Cl bands at the metal interface in specimens with and without TCF in seawater solution can be attributed to the attraction between Cl− ions and the positive charge of the metal surface, resulting in the formation of iron(III) chloride83. The Cl 2p peak was detected at 197.46 eV and 198.96 eV for the non-TCF specimen and at 198.48 eV and 200.05 eV for the TCF specimen due to the presence of Cl 2p3/283. The XPS profile of iron-2p was shown in the prominent peaks at around 710 eV and 724 eV, which were associated with Fe-2p3/2 of Fe(II)85,86. In addition, the O–1 s profile at peaks of 530 eV and 528 eV was suggested to be related to the O and OH–atoms bonded to Fe(II) and Fe(III) to form FeO/Fe2O3 and FeOOH oxides, respectively87.
The shift in binding energy in the XPS spectrum can reveal changes in the oxidation state of iron and the formation of iron oxide or hydroxide species, indicating corrosion10. The presence of new peaks corresponding to functional groups from the TCF indicates that these compounds interact chemically with the steel surface, further increasing corrosion resistance88.
The morphology and surface analysis results confirmed that TCF significantly enhanced the corrosion resistance of carbon steel in a simulated seawater medium. This finding was confirmed by the formation of a protective layer by bioactive compounds from TCF through the adsorption process to the steel surface, and a decrease in surface roughness and smoother surface morphology on the steel surface added with inhibitors, as well as the presence of chemical interactions between the inhibitor and the steel surface that can improve corrosion resistance.
Computation analysis
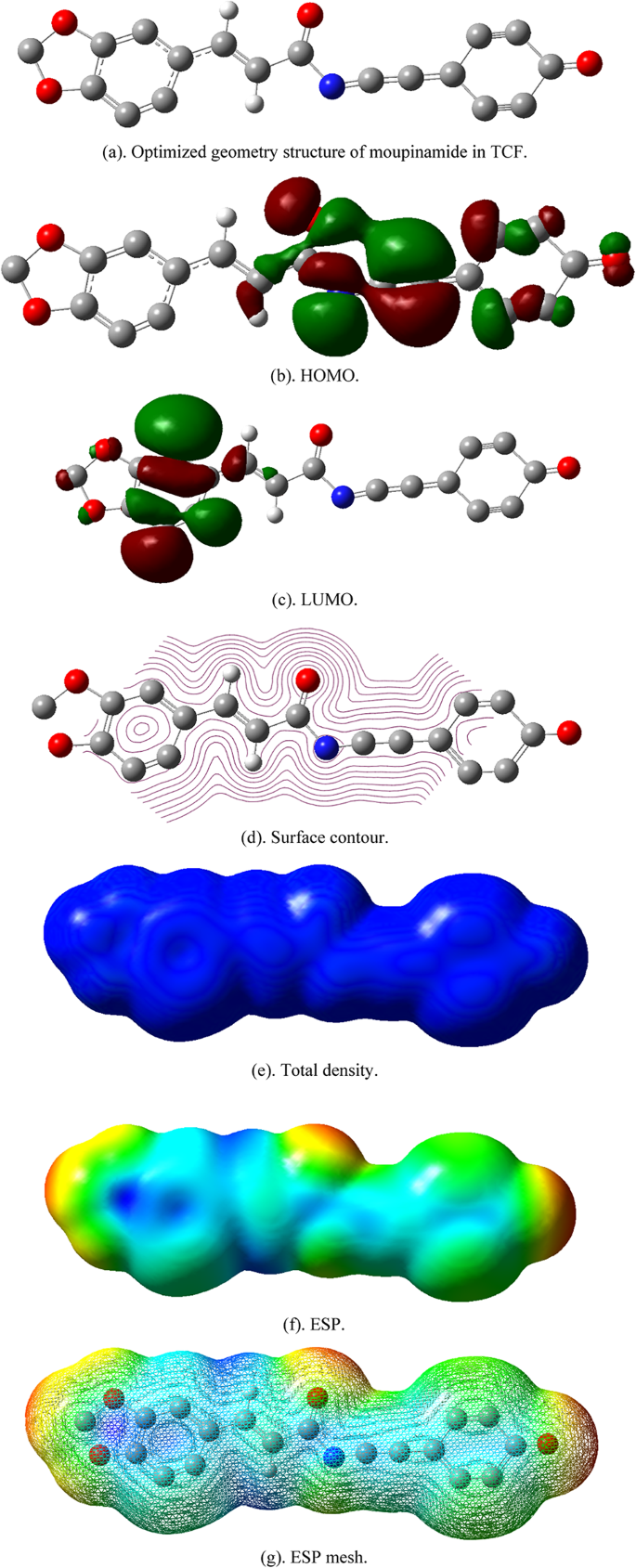
Experimental measurements have demonstrated that the bioactive compound in TCF (moupinamide) exhibits strong corrosion inhibition efficiency, with a protective efficacy exceeding 90% at a concentration of 150 mg.L−1. To model and comprehensively understand the electronic properties of moupinamide and its interaction with the carbon steel surface, computations using density functional theory (DFT) were carried out. The results of the structure optimization, including the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), surface contour, total density, and electrostatic potential (ESP), are depicted in Fig. 15, along with detailed data of quantum chemical parameters tabulated in Table 8. HOMO describes the region that tends to donate electrons, while LUMO indicates the main electron-accepting region in a molecule42. As seen in Fig. 15, the electron distributions of HOMO and LUMO are primarily concentrated in the aromatic ring and N-trans double bond compounds, indicating their high ability in electron exchange and interaction with metal surfaces. According to the DFT results, for the bioactive compounds in TCF, the EHOMO value was negative, revealing that the physical adsorption is more probable than chemical adsorption. Physical adsorption has been described in various scientific articles as expressed as a negative EHOMO1,8,30.
Optimized geometry structure, HOMO, LUMO, surface contour, total density, and ESP for moupinamide from TCF in NaCl medium. The molecular structure and electronic properties were modeled using BIOVIA Materials Studio 2020 and GaussView 6.0.16 with the Dmol3 module under GGA-PBE functional and DNP basis set. The HOMO and LUMO orbitals were localized mainly on the aromatic and amide functional groups, indicating high reactivity and interaction potential with carbon steel surfaces.
Table 8 List of the parameters attained through the DFT analysis.
The modeling and calculation results show that the ΔE, IP, and EA values are 0.08, 0.28, and 0.19 eV, respectively. The smaller HOMO–LUMO energy gap (ΔE = 0.08) indicates that electron donation from the inhibitor to the metal surface (reactive compound) is easier, which enhances its inhibitory ability. The calculated energy gap of moupinamide indicates favorable electronic properties for corrosion inhibition. A stronger synergistic effect of the inhibitor with the Fe metal corresponds with a reduced ΔE value6. The electron density distribution reveals that the nitrogen and oxygen atoms in the structure have significant electron donation abilities, which are essential for interacting with the metal surface80. In this work, the calculated energy gap of moupinamide suggests advantageous electronic characteristics for a corrosion inhibitor. The IP and EA values indicate that TCF can act as an electron donor and acceptor.
The computational and calculation results also confirmed that TCF has a lower chemical hardness (η, 0.23 eV) and tends to be soft (σ, 4.2 eV), indicating that the molecule is reactive and able to interact with metals. Chemical hardness (η) refers to the resistance of chemical species to polarization or deformation of electron clouds, where the inhibition efficiency is inversely proportional to the chemical hardness of a compound89. σ and η are essential factors that provide information regarding reactivity and stability, which could affect the efficacy of an inhibitor19. Soft molecules with small HOMO–LUMO energy gaps can be used as corrosion inhibitors because they have low HOMO–LUMO energy gaps17. In addition, ∆N denotes the release of electrons to the vacant orbital. The capacity of a chemical to inhibit is enhanced when its value is positive. The bioactive compound of TCF exhibited a positive value of 0.11, implying increased stability and chemical reactivity.
Based on the ΔE, σ, and η values, combined with the ∆N, it indicates that the TCF results are effective in reducing corrosion on carbon steel surfaces. Furthermore, the results of the electrostatic potential (ESP) map and ESP mesh tend to be evenly distributed throughout the molecule, with surrounding NaCl ions. The interaction with NaCl can slightly distort the geometry of the molecule, affect the electronic structure (HOMO/LUMO), and change its surface properties (ESP). These changes can affect the chemical behavior and interactions of moupinamide in the environment, which may be essential for further research on its potential use in developing science materials.