Diagnosis
Our patient, a 56-year-old Caucasian male with type 2 diabetes was diagnosed with non-secreting MM. Initially, he presented with a low-energy fracture of the right clavicle in April 2019. He had no history of tobacco use, and there was no indication of any chronic pulmonary disease in the patient’s medical record. Computed tomography (CT) and magnetic resonance imaging (MRI) confirmed the pathological nature of the fracture and the presence of osteolytic lesions in other regions of the skeleton. Laboratory examinations revealed mild macrocytic anemia, normal renal function, borderline hypercalcemia, and elevated beta-2-microglobulin levels. However, serum protein electrophoresis showed no monoclonal band and only slight hypogammaglobulinemia. Serum and urine immunofixation did not show any monoclonal protein. The test for free light chains in serum was slightly abnormal, with a kappa-to-lambda ratio of 3.92. The bone marrow cytology revealed a 25% infiltration by plasma cells with abnormal morphology, whereas trephine biopsy showed almost 90% involvement by pathologic plasma cells (kappa+ , lambda−, CD20+ in the subset of cells). Fluorescence in situ hybridization (FISH) examination excluded the TP53 gene deletion and confirmed the presence of t(11; 14) translocation in 85% of examined cells. On the basis of the above, in August 2019, a diagnosis of nonsecreting multiple myeloma with t(11; 14) translocation was established.
TreatmentFirst-line treatment
Owing to the absence of therapeutic guidelines designed specifically for nonsecreting multiple myeloma, a standard first-line regimen was adopted based on bortezomib in combination with lenalidomide and dexamethasone (VRD). After four courses, bone marrow cytology revealed 1.4% plasma cells. In the subsequent positron emission tomography (PET)/CT evaluation, metabolically inactive osteolytic changes within the bone were observed, characterized by diminished size and marginal sclerotization. In January 2020, he underwent autologous hematopoietic stem cell transplantation (HSCT). Consolidation treatment included another four courses of VRD regimen. Treatment resulted in complete response (CR) with detectable minimal residual disease in bone marrow (MRD). Lenalidomide 10 mg was administered continuously as maintenance treatment.
Second-line treatment
After 1 year of maintenance and 18 months after HSCT, in July 2021, disease relapse was diagnosed with dynamically increasing marrow involvement and moderate pancytopenia, without new FISH abnormalities and new bone lesions. After reviewing available data from literature, to address early aggressive relapse with lenalidomide resistance, the second-line treatment comprised daratumumab, carfilzomib, and dexamethasone (DKd). Baseline cardiac function assessment revealed no contraindications to treatment. Carfilzomib was scheduled at a dose of 70 mg/m2 intravenously once weekly, on days 1, 8, and 15 of each 28-day cycle, with an initial test dose of 20 mg/m2. Once-weekly dosing instead of a typical twice-weekly schedule was chosen according to the ARROW study [14]. Beginning with the second cycle, daratumumab was added, in the form of subcutaneous injections of 1800 mg, every week for 8 weeks, then every 2 weeks in cycles 3–6, and every 4 weeks thereafter (dosing as in the CANDOR study) [5]. After three courses of treatment, the bone marrow response was assessed, and CR was confirmed with positive MRD at the level of 0.007% (multicolor flow cytometry).
Pulmonary toxicity
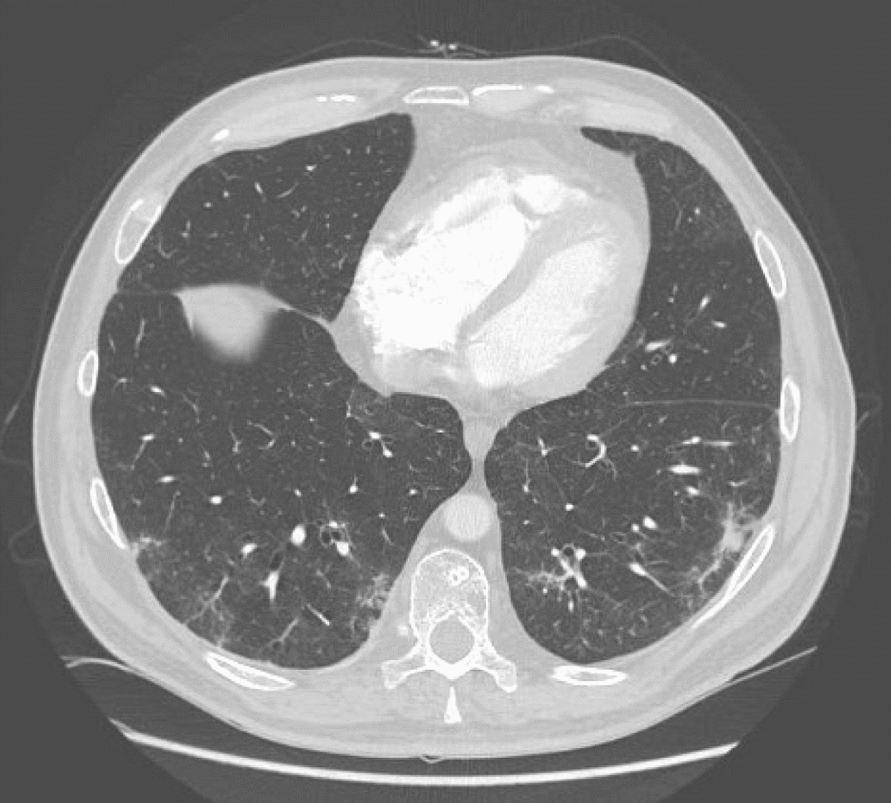
At the beginning of the third cycle of DKd, the patient reported elevated temperature and mild shortness of breath lasting 1–2 days following the administration of chemotherapy, resolving without medication. In March 2022, after six cycles of DKd, the patient developed severe dyspnea, tachycardia, and pyrexia with somnolence. The symptoms started 24 h after carfilzomib dose. Oxygen saturation dropped below 90%. The patient was admitted to hospital. Blood biochemistry showed normal kidney and liver function, and troponin I, C-reactive protein (CRP), d-dimers, and other coagulation tests were in reference ranges. The only aberration was elevated N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) (370 pg/ml; reference range < 125 pg/ml). Thoracic angio-CT showed no signs of pulmonary embolism. However, peripherally in the lower lobes, interstitial and reticular opacities with areas of consolidation were seen as well as ground-glass opacities. Similar smaller lesions were also found subpleurally in the second segment and the middle lobe of the right lung and in the lingula of the left lung (see Fig. 1). In addition, transthoracic echocardiography showed maximal tricuspid velocity of 2.9 (m/s), indicating a moderate probability of significant elevation of pulmonary arterial pressure; no other signs of cardiac dysfunction were noted. In April 2022, the diffusing capacity of the lungs for carbon monoxide test (DLCO) and plethysmography were ordered. The DLCO at 69% was below the reference range, while no signs of volume restriction nor bronchial obturation were present.
Axial slice of contrast-enhanced high-resolution computed tomography scan depicting subpleural peripheral reticular opacities. Lytic lesion in the left aspect of the T10 vertebral body
As mentioned before, the patient did not report any pulmonary symptoms before starting therapy. The episodes of dyspnea and pyrexia occurred only in close temporal connection with carfilzomib administration. Notably, the symptoms were repeatedly present after each carfilzomib dose, irrespective of concomitant daratumumab dosing, which was given every other week. After careful analysis of the medical records, adverse effect of carfilzomib was suspected and treatment was withheld. The patient’s status improved. Daratumumab as monotherapy was continued according to the previous schedule, without reappearance of pulmonary symptoms. Repeated DLCO measurement after 2 months showed partial improvement at 72%, whereas high-resolution lung CT documented partial regression of interstitial and reticular lesions (see Fig. 2).
Axial slice of non-contrast computed tomography scan acquired 2 months after initial study. Almost complete regression of previously observed lung abnormalities
After the next 3 months, in July 2022, pulmonary symptoms completely subsided, and carfilzomib rechallenge was attempted. Carfilzomib dose was reduced to 56 mg/m2. However, after two doses of the drug, pyrexia up to 40 ℃ reappeared, accompanied by pleural pain and decreased oxygen saturation, peaking between 24 and 48 h after drug administration. Again, carfilzomib cessation led to complete resolution of symptoms. The patient remained on daratumumab maintenance with good tolerance and response to treatment. Drug-related complications were immediately reported to the relevant regulatory office in Poland.