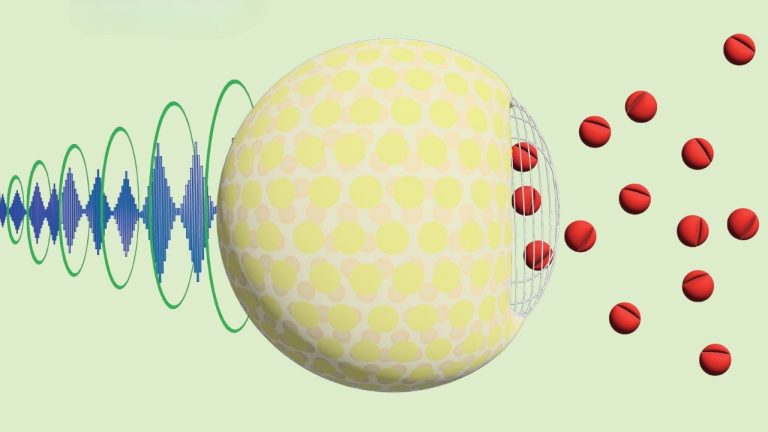
After testing different types and concentrations of sugars, the researchers found that 5% sucrose added to the liquid core achieved the best balance of ultrasound response and stability at body temperature. More sugar could make the nanoparticles even more responsive to ultrasound, but it also increased the drug leakage without ultrasound.
The mechanism of how ultrasound causes drug release is still unclear. The researchers think the ultrasound oscillates the surface of the nanoparticles against the denser core, creating pores that release the drug.
Hitting the target
The researchers then tested the drug delivery system in rats, comparing animals who were given an injection of free, unencapsulated ketamine with those given ketamine encapsulated in nanoparticles with 5% sucrose. Without any application of ultrasound, the rats that received the nanoparticles had less than half the amount of ketamine in every organ the researchers tested.
“We looked at the brain, liver, kidney, spleen, lung, heart and spinal cord — and wherever we had sufficient ability to detect it, we saw less ketamine with the liposomal formulation,” Airan said.
When the researchers applied ultrasound to a particular brain region, the nanoparticles delivered about three times as much drug to that region as to other parts of their brain — demonstrating targeted drug release.
Though the targeted brain area received only about 30% more ketamine from the nanoparticles than from free ketamine, the selectivity of the increase made a significant difference to brain function.
“It’s not just that we’re getting the on-target effect. We’re getting more of it than what you might expect, based on how much we’re delivering to that part of the brain,” Airan said.
The researchers found they could decrease anxious behavior in rats by targeting ketamine release to their medial prefrontal cortex, the brain region that controls emotional states. Rats that received the targeted treatment spent more time roaming the center of a box — a sign of less stress — compared with counterparts that received free ketamine or a saline control.
If the system works in humans, clinicians may be able to isolate the emotional effects of ketamine — to treat depression, for example — while blocking the dissociative effects of the drug.
Pain point
The researchers also demonstrated they could block pain in a specific part of the body by targeting a local anesthetic, ropivacaine, to a specific nerve. When researchers gave rats ropivacaine encapsulated in nanoparticles and applied ultrasound to the sciatic nerve in one leg, that leg would become numb to a prick. A 2.5-minute ultrasound session induced local anesthesia for at least an hour.
Such a procedure would have another advantage for patients in pain. Typically, local anesthetics require an injection at the source of the pain, which can add to a patient’s discomfort. With the new system, the drug can be injected elsewhere as an ultrasound is applied non-invasively at the site of the pain.
Pending a greenlight by the U.S. Food and Drug Administration, Airan’s team is planning the first human trial of the ultrasound drug delivery system, which will use ketamine to target a patient’s emotional experience of chronic pain.
A few years ago, when Airan approached pharmaceutical companies about producing the earlier version of the nanoparticles, the calls would end when they heard about the exotic ingredients. This time around, a tiny spoonful of sugar has made the pitch more palatable. “It’s eminently translatable,” he said.
The study received funding from the National Institutes of Health (grants RF1MH114252, UG3NS114438 and UG3NS115637), the Stanford Wu Tsai Neurosciences Institute, an anonymous donor to the Stanford Medicine Department of Radiology, the Ford Foundation and the National Science Foundation.