Our pharmacovigilance study represents the first comprehensive analysis of triptorelin’s post-marketing safety profile using FAERS data. The identificationof novel signals, including defiant behavior and Alzheimer’s dementia, suggests areas requiring further investigation, though these associations should be interpreted as hypothesis-generating rather than conclusive evidence of causality. Before considering updates to the Summary of Product Characteristics (SmPC), rigorous clinical validation through controlled prospective studies is essential. Notably, several detected adverse events may reflect underlying conditions rather than direct drug effects. The strong statistical signal for Alzheimer’s dementia (ROR = 68.90) was observed predominantly in patients > 64 years, a population with inherent dementia risk. Confounding by age cannot be excluded. For signals like death in prostate cancer patients, disease progression remains the predominant explanation. Disentangling drug effects from underlying malignancy requires studies with matched comparators.
The gender distribution of AEs(45.55% females vs. 45.84% males) aligns with triptorelin’s indications for prostate cancer(males)2,4 and endometriosis(females)1,5,9. Additionally, patients under 18 years old accounted for a relatively high proportion (18.22%), consistent with the indication of triptorelin for children 2 years and older with central precocious puberty (CPP)3. Notably, we observed an increasing trend in AE since 2019, suggesting both expanded clinical use and heightened pharmacovigilance awareness. While 49.98% of reports came from consumers(often with incomplete data), 47.09% originated from medical professionals (including health professionals, physicians, other health professionals, and pharmacists), providing more reliable safety information.
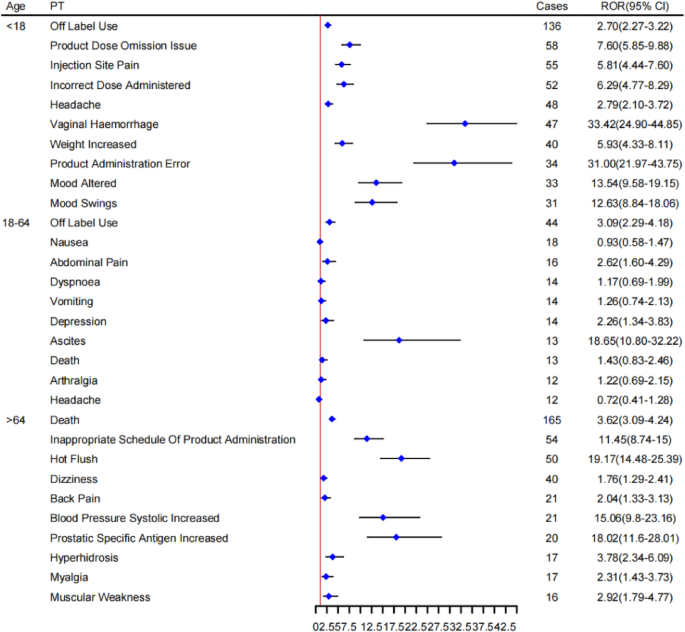
Age-stratified subgroup analysis of AEs reported with triptorelin.
Disproportionality analysis identified “reproductive system and breast disorders” and “product issues” as the most prevalent statistically significant signals at the SOC level. Product Issues likely reflect use-related factors (e.g., handling errors or misinterpretation of adverse event descriptions) rather than direct drug effects. Reproductive system and breast disorders signals (e.g., vaginal haemorrhage, breast enlargement, ovarian hyperstimulation syndrome, dysmenorrhoea, testicular atrophy, dyspareunia) align with established triptorelin pharmacology and labeling information. Mechanistically, transient sex steroid hormone surges within 2–4 weeks post-dose may induce puberty-like symptoms (e.g., vaginal bleeding)28,29, corroborated by clinical observations (incidence: 4.6% in one cohort29.
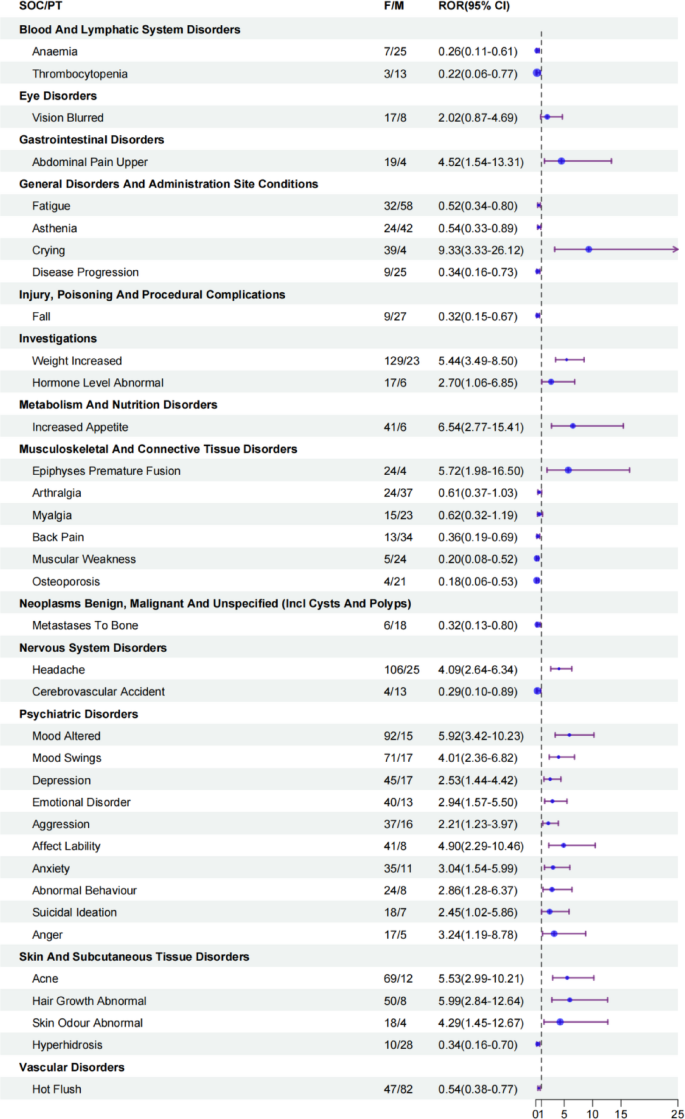
Sex-stratified disproportionality signals of triptorelin-associated AEs. CI, confidence interval; ROR, reporting odds ratio.
Our analysis confirmed triptorelin’s established safety profile through statistically significant signals of expected adverse events (AEs) aligned with its pharmacological mechanism. Vasomotor symptoms dominated the AE spectrum, with hot flushes reported in 71.7% of triptorelin embonate recipients2,4 and 75% in 3-month formulations1, consistent with GnRH agonist-induced hypoestrogenism. Other label-congruent signals included sexual dysfunction (testicular atrophy: 7.5%2,4, reproductive disorders (vaginal haemorrhage, ovarian hyperstimulation syndrome), and metabolic effects (weight increase, hormone abnormalities). These AEs typically manifested within the initial treatment month, were generally self-limiting, and rarely warranted therapy discontinuation6,7,8,9, reinforcing current risk management strategies.
Beyond expected effects, disproportionality analysis detected 73 potential safety signals (102 statistically significant PTs), stratified as 36 strong (IC < sub > 025 > 3.0) and 23 medium-strength signals (1.5 < IC < sub > 025 ≤ 3.0). Crucially, 21 strong signals represented previously unreported associations (Table 2). Particularly noteworthy were 21 strong signals representing previously unreported associations that warrant careful evaluation. The detection of Alzheimer-type dementia as a strong signal requires special consideration given the potential confounding by age (21.65% of triptorelin users were > 64 years) and possible misattribution of baseline dementia prevalence in this population. Similarly, signals for psychiatric and behavioral adverse events, including the notably strong signal for defiant behavior in pediatric populations, may reflect either true neuroendocrine effects of GnRH agonists or underlying behavioral conditions in treated populations like central precocious puberty.
These novel signals, while requiring validation for causal inference, highlight important potential safety concerns across multiple systems. The neuropsychiatric signals (Alzheimer-type dementia, personality changes), endocrine/growth effects (altered height progression, testosterone decreases), and serious systemic events (pituitary apoplexy, acute coronary syndrome) all merit targeted surveillance and mechanistic investigation. Particularly urgent is the need for rigorous epidemiological studies to evaluate the dementia signal while controlling for age-related confounding, as well as focused research on neuropsychiatric outcomes in pediatric populations receiving GnRH agonists. The biological plausibility of these associations should be explored while considering the limitations inherent in spontaneous reporting data.
In Lundstrom’s study, among 120 patients, 115 (95.8%) reported treatment- emergent AEs. 17 patients reported a serious adverse event (SAE), three of whom expired during the study: two succumbed to prostate cancer progression at month 7 and month 8, notwithstanding maintained castration, and a third patient, who was at high risk for coronary occlusion, died of myocardial infarction at month 4. All deaths were adjudged as unrelated to the study drug. Additionally, clinically significant treatment-emergent laboratory abnormalities were observed in nine patients; only two mild events in the same patient were deemed to have a relationship with the study drug. These were increased ALT (to 60 U/L on day 337 [normal range 6–48 U/L]) and AST (to 65 U/L on day 337 [normal range 10–45 U/L])2. In our study, an unexpected mild safety signal, hepatitis fulminant, was also obtained. It is essential to counsel physicians or pharmacists to monitor laboratory abnormalities during triptorelin treatment.
The analysis suggests that reports of AEs predominantly occurred within one month. (22.59%) or after one year (25.07%). Consequently, vigilance for these statistically associated AEs may be warranted pending further validation both during the first month and beyond one year. Early detection and management of AEs reported with triptorelin are critical. Notably, comprehensive studies on the precise timing of post-administration adverse reactions are lacking, highlighting the value of our investigation. However, TTO analysis was only feasible for 18% (726/4018) of primary suspect triptorelin reports due to missing or invalid date information. This substantial missing data proportion compromises the generalizability of TTO findings (including median, IQR, and temporal distribution) and introduces potential selection bias. Therefore, these results should be interpreted cautiously, primarily reflecting characteristics of reports with complete temporal data.
Triptorelin is a gonadotropin-releasing hormone (GnRH) agonist. Its mechanism of action involves continuous stimulation of pituitary gonadal hormones, leading to receptor down regulation, desensitization of gonadotropin cells, and suppression of gonadotropin (luteinizing hormone, follicle-stimulating hormone), resulting in diminished sex steroid production28,30. Gender differences in pharmacokinetics may contribute to variations in the reported AE profiles between males and females31,32. However, there is a paucity of reported gender-specific AEs associated with triptorelin treatment. In our study, we noted that females exhibited a marginally higher number of positive signal values for AEs compared to males. Some of the AEs with strong statistical signals more prone to occur in women included upper abdominal pain, crying, weight increased, hormone level abnormal, increased appetite, epiphyses premature fusion, headache, psychiatric disorders, acne, abnormal hair growth, and abnormal skin odour. Such gender-specific reporting patterns may inform pharmacovigilance monitoring, efficacy, and optimizing drug therapy for both genders.
Furthermore, in the age subgroup analysis, some preferred term (PT) specific to each age stage were identified. For instance, vaginal haemorrhage, weight increased, and mood altered signals were present in the subgroup of age < 18; abdominal pain and depression signals were in the subgroup of age 18–64; and hot flush, back pain, increased systolic blood pressure, prostatic specific antigen increased, and muscular weakness signals were in the subgroup of age > 64. Studies have cited that differences in pharmacokinetics with advancing age may either augment or attenuate differences in drug response33. The result of age-specific AEs will also assist in augmenting the safety and effectiveness of drugs in specific populations, such as children and the elderly.
While our analysis identified significant associations between triptorelin and adverse event reporting in FAERS, several key limitations of spontaneous reporting systems must be emphasized to avoid misinterpretation.
Firstly, reporting biases inherent to voluntary systems may distort findings: novel AEs (e.g., defiant behavior) are more likely to be reported due to their unexpectedness, while common labeled events (e.g., hot flashes) may be underreported, potentially skewing signal strength. Additionally, temporal variations in reporting (the Weber effect) and media influence can further complicate signal detection, introducing inconsistencies in how events are captured over time.
Secondly, the selection of all non-triptorelin FAERS reports as the comparator group, while consistent with standard disproportionality analysis methodology for initial signal detection16,17, presents inherent limitations. This approach does not adequately adjust for fundamental differences in patient demographics (e.g., 21.65% elderly users), underlying disease profiles (84.3% malignancy indications), or indication-specific reporting patterns that characterize triptorelin users. To enhance signal specificity in future research, active comparator designs utilizing other GnRH agonists (e.g., leuprolide) would be preferable, as their shared mechanism of action and similar patient populations would better control for confounding by indication.
Thirdly, critical methodological constraints persist: the absence of a defined denominator (total number of triptorelin users) precludes calculating incidence rates, limiting our ability to quantify actual risk34. Unmeasured confounders—including comorbidities, polypharmacy, and disease severity—further hinder efforts to isolate triptorelin-specific effects, while channeling bias (e.g., preferential use in advanced cancer stages) may introduce additional confounding. Notably, confounding by underlying diseases may distort signals: for example, bone pain in prostate cancer patients could reflect tumor metastasis rather than triptorelin. Concomitant medications (e.g., opioids for cancer pain) might also interact with triptorelin, potentially amplifying musculoskeletal AEs, which we could not disentangle due to data limitations.
These limitations underscore that our findings are hypothesis-generating rather than conclusive. Disproportionality analysis effectively identifies statistical signals but cannot establish causality35. The results should instead prompt enhanced clinical vigilance and validation through robust epidemiological designs, such as prospective studies, to confirm these associations and clarify their clinical significance.