The use of advanced MRI techniques in the study of patients diagnosed with an IGE has been scarce, particularly with regards to regional brain segmentation and volumetric assessment. In our study, we conducted a comprehensive evaluation of numerous cerebral regions in patients with IGE, comparing them to a group of normal controls.
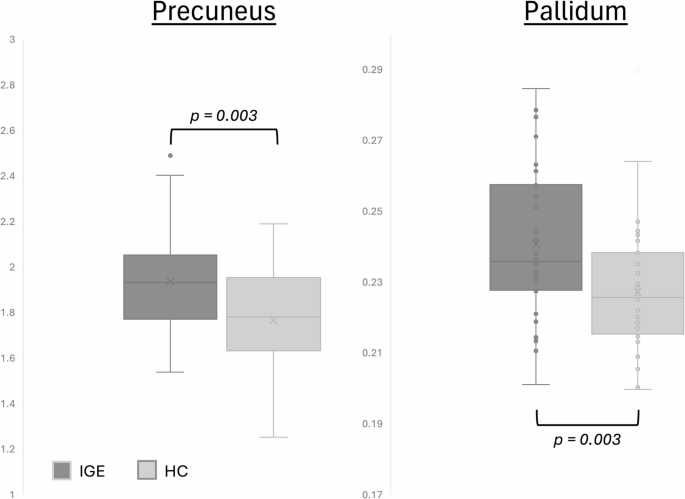
Our findings revealed a significant increase in the normalized volumes of the precuneus in patients with IGE compared to the control group. The precuneus is anatomically located in the postero-medial portion of the parietal lobe, anterior to the cuneus of the occipital lobe from which it is separated by the parieto-occipital fissure. It is also positioned posterior to the paracentral lobule, and superior to the sub-parietal sulcus20. It includes both unimodal and polymodal association regions and plays a crucial role in visual-spatial imagery, sensorimotor functions, and consciousness21. Precuneal epilepsy is rarely reported in the literature due to its diverse heterogeneous presentations and complex circuitry20. Previous studies have implicated the precuneus in the pathogenesis of IGE, as reduced functional connectivity has been observed in this region, as well as in the posterior cingulate gyrus19. This highlights the importance of further investigating and understanding the seizure pathophysiology in IGE patients, which differs significantly from other classic epilepsy syndromes3. The functional consequences of increased precuneus volume in IGE remain to be fully elucidated. However, given the region’s involvement in the default mode network (DMN)—which governs consciousness, introspection, and attention—alterations in its structure may reflect compensatory neuroplastic responses or disrupted synaptic pruning. These changes could influence network excitability and impair the dynamic regulation of attention and awareness during seizures6,22. The observed volumetric increase may thus represent a structural correlate of altered connectivity and dysfunctional consciousness mechanisms in IGE.
We also observed a significant increase in normalized volumes of the pallidum in patients with IGE compared to the control group. This finding contrasts with previous studies that reported reductions in the volume of the globus pallidus3,23. The discrepancies between our findings and those of other studies may be attributed to differences in sample sizes (38 IGE cases in our study compared to 14 IGE cases in the study by Du et al.23. The basal ganglia, including the globus pallidus, have been suggested to modulate the occurrence of spike-wave discharges in IGE through feedback circuits to the thalamus and cerebral cortex. It has been also suggested that abnormally increased activity in subcortical structures including the basal ganglia may play a crucial role in the motor manifestations of IGE24.
In our study, we did not find a statistically significant increase in the volume of the subcallosal cortex of patients with IGE compared to normal controls. This finding contrasts with a VBM study that reported an increase in GM volume in the right subcallosal gyrus in patients with absence epilepsy25. However, considering that reduced connectivity and function of the subcallosal gyrus have been implicated in treatment-resistant major depression26, further evaluation of this often-overlooked region may be warranted in patients with IGE.
We did not observe any statistically significant differences in the volume in the precentral gyrus, or frontal gyri between patients with IGE and normal controls3. The precentral gyrus, also known as the primary motor cortex, is responsible for executing voluntary movements on the contralateral side of the body. Previous studies investigating the function and involvement of the precentral gyrus in IGE have not yielded conclusive results, highlighting the need for further neurofunctional studies to explore the correlation between dysfunctional motor symptoms during seizures and activation of this specific brain region27.
It is important to acknowledge a few limitations to our study: the use of MRI data acquired from two different scanners with varying parameters, including field of view, spatial resolution, and flip angles, and the lack of standardized MRI protocols across imaging centers. However, this limitation was minimized through a consistent preprocessing pipeline—including bias correction and non-linear registration—and by performing visual quality control and manual correction of all segmented regions. Additionally, volumetric analysis was conducted using the same software for all included patients, after excluding non-diagnostic or sub-diagnostic evaluations. While these steps reduce potential bias, future studies may benefit from the integration of harmonization approaches or scanner-adjusted modeling. Considering that previous studies focusing on diffusion kurtosis have revealed distinct patterns of microstructural alterations in patients with IGE, particularly involving thalamo-cortical projections28, we suggest that future studies combining diffusion kurtosis and volumetric analysis may provide further insights into these findings.
Although this study did not include an a priori power calculation, a post hoc sensitivity analysis using GPower (α = 0.05, power = 0.80, two-tailed t-test) indicated that the sample size was adequate to detect medium to large effect sizes. The significant findings in the precuneus and pallidum volumes fall within this range, supporting the robustness of the observed group differences.
The observed volumetric increases in the precuneus and pallidum in IGE patients raise important considerations for clinical practice. Although our study is cross-sectional and does not directly assess clinical outcomes, previous work suggests that such structural alterations may hold predictive value. For example, increased precuneus volume has been associated with altered default mode network activity, which may influence attention and consciousness during seizures6. Similarly, basal ganglia abnormalities, particularly involving the pallidum, have been linked to motor manifestations and seizure modulation through thalamocortical loops5. These findings support the potential of regional volumetric markers as complementary tools for identifying IGE subtypes with distinct clinical or cognitive profiles. Future studies may investigate whether these morphometric features correlate with pharmaco-resistance, cognitive deficits, or long-term seizure control, thereby informing personalized treatment strategies.
Although this study did not assess cognitive or motor performance directly, previous work has linked abnormalities in the precuneus to deficits in attention, introspection, and working memory among patients with IGE6. Increased volume in this region may reflect altered neurodevelopmental pruning or compensatory reorganization in response to seizure-related network disruption29. Similarly, the pallidum is involved in the modulation of motor output and seizure propagation through basal ganglia-thalamocortical circuits30. Structural changes in this region may be associated with motor manifestations, particularly in patients experiencing GTCA5. Future studies combining volumetric analysis with neuropsychological and motor assessments could clarify the clinical impact of these findings.
In our study, the decision to consolidate the IGE subsyndromes offered several advantages such as the increase in sample size, a better statistical power to identify generalizable trends and shared morphometric patterns with potential clinical implications across IGE patients. However, it also limited our ability to isolate subsyndrome-specific characteristics. Therefore, it is important to recognize the trade-off between broader insights and the depth of subsyndrome-specific exploration within our study context.