Characterization
In this research, Sol nanomicelles incorporating Vn were synthesized via the thin film method for integration into 3D-printed scaffolds. Figure 1 illustrates the FESEM image of these nanomicelles. As observed, the Sol nanomicelles containing Vn exhibited a perfectly spherical morphology, with no indications of agglomeration or aggregation. Based on image, their average diameter was determined to be 80.2 ± 11.9 nm. A similar structural characteristic was reported in a related study by Piazzini et al.16 for Sol-based nanomicelles.
FE-SEM image of Vn-loaded Sol-based nanomicelles.
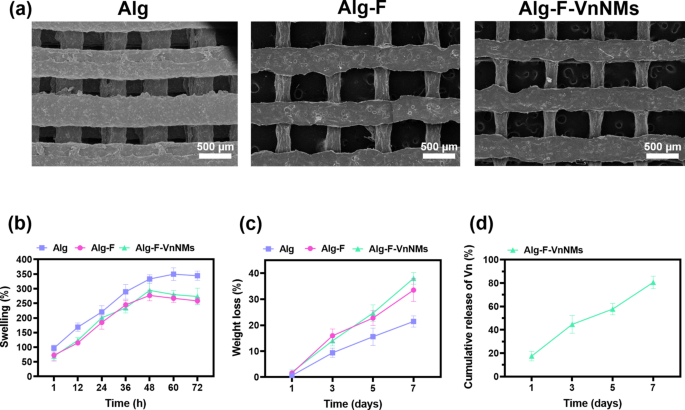
SEM images of the 3D-printed scaffolds are presented in Fig. 2a. These mesh-like structures are fabricated through a repetitive arrangement of perpendicular strands across multiple layers. All scaffolds possess open and interconnected channels and demonstrate a relatively uniform morphology. The strand diameters measured for the Alg, Alg-F, and Alg-F-VnNMs scaffolds were 413.7 ± 26.4 µm, 289.2 ± 64.2 µm, and 275.2 ± 82.1 µm, respectively. Also, the channel diameters for these scaffolds were determined to be 369.8 ± 37.0 µm, 684.5 ± 28.8 µm, and 656.2 ± 44.1 µm, respectively. Consequently, scaffolds incorporating F exhibited significantly thinner strands and wider channels compared to the Alg scaffold (p < 0.001). This effect is likely due to an increase in the polymer solution concentration and the viscosity of the printing ink following the addition of F. As the viscosity of the 3D printing ink rises, the nozzle output diminishes under constant pressure, leading to the formation of thinner strands and larger channels29. It is worth noting that the addition of nanomicelles did not cause a significant change in the structure of the Alg-F-VnNMs scaffold compared to the Alg-F scaffold.
(a) SEM images showcasing the microstructure of the fabricated scaffolds, (b) swelling behavior of the scaffolds in distilled water, (c) weight loss of the scaffolds due to degradation in PBS, and (d) release profile of Vn from the Alg-F-VnNMs scaffold in PBS.
Wound exudates, the secretion of tissue fluid following an injury, facilitate the transfer of biochemical substances and foster a suitable environment for wound recovery. However, an overabundance of wound exudates can extend the inflammatory phase and impede recovery, especially in cases of chronic wounds30. The swelling behavior of the fabricated scaffolds was evaluated based on their water uptake ability over 72 h (Fig. 2b). All scaffolds exhibited a significantly increasing swelling trend in a similar manner, with the distinction that equilibrium swelling was achieved for the Alg scaffold after 60 h and for the Alg-F and Alg-F-VnNMs scaffolds after 48 h. Accordingly, the highest swelling rate was measured at 349.7 ± 21.6% for the Alg scaffold, while for the Alg-F and Alg-F-VnNMs scaffolds, the swelling rates were 277.0 ± 18.1% and 294.3 ± 24.1%, respectively. The significantly higher swelling capacity of the Alg scaffold compared to the others may be attributed to its thicker strands and greater water absorption ability. After reaching equilibrium swelling, the swelling of the Alg scaffold remained nearly constant (with a slight decrease) until 72 h, whereas the swelling of the Alg-F and Alg-F-VnNMs scaffolds noticeably declined until 72 h. This reduction may be due to the degradability of these scaffolds affecting the swelling rate.
Biodegradability
A skin tissue scaffold must maintain sufficient stability to support the adhesion and colonization of native cells across its surface and within its structure, while degrading at an appropriate rate. This controlled degradation facilitates the release of loaded molecules and enables the regeneration of native tissue in a sequential manner31. According to Fig. 2c, all fabricated scaffolds experienced a progressive weight reduction over 7 days of immersion in PBS. The degradation of the Alg, Alg-F, and Alg-F-VnNMs scaffolds after 7 days was measured to be 21.4 ± 2.1%, 33.5 ± 4.4%, and 38.0 ± 2.25%, respectively. Based on the results, the incorporation of F and Sol-based nanomicelles into the Alg scaffold led to an increase in biodegradability. Following the cross-linking of the scaffolds, covalent cross-links between calcium ions and guluronic acid blocks in alginate enhanced the stability of the scaffolds32, however, F and nanomicelles, as additives, did not participate in such linkages; therefore, the degradation rate of the Alg-F and Alg-F-VnNMs scaffolds was higher than that of the scaffold made from pure Alg.
Vn release profile
In this study, the encapsulation of Vn in Sol nanomicelles was carried out to achieve stability and optimal dispersion of Vn in the hydrophilic Alg-based solution. The encapsulation efficiency of Vn in Sol nanomicelles was measured to be 64.8 ± 3.9%. Figure 2d represents the release profile of Vn from the Alg-F-VnNMs scaffold. The release of Vn on the first day was recorded as 17.5 ± 4.1%. Following a sustained manner, the release of Vn reached 80.6 ± 5.3% by day 7. The two primary factors contributing to the release of Vn from the Alg-F-VnNMs scaffold were degradation and swelling, accompanied by the elution of nanomicelles. A controlled and sustained release of the loaded antibacterial agent within the skin tissue scaffold at the wound site is considered a clinical advantage, as it enhances bioavailability and effectiveness while reducing side effects33.
Assessment of antibacterial efficacy
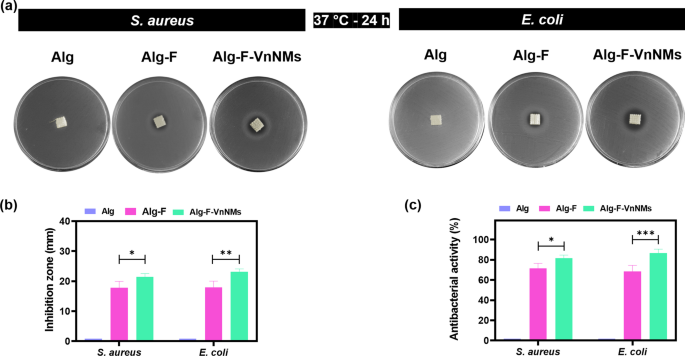
The antibacterial properties of the fabricated scaffolds were examined through the inhibition zone method and bacterial viability assessment. Figure 3a displays images of the inhibition zones formed by different scaffolds against S. aureus and E. coli. As observed, the Alg scaffold, due to its lack of antibacterial properties, did not produce any inhibition zone against the bacteria. In contrast, the Alg-F and Alg-F-VnNMs scaffolds led to inhibition zones of 17.8 ± 2.1 mm and 21.4 ± 1.15 mm against S. aureus, and 17.9 ± 2.0 mm and 23.2 ± 0.9 mm against E. coli, respectively (Fig. 3b). Figure 3.c depicts the antibacterial activity of different scaffolds in contact with S. aureus and E. coli. Consistent with the results of the inhibition zone test, the Alg scaffold exhibited no antibacterial properties against the bacteria, whereas the Alg-F and Alg-F-VnNMs scaffolds demonstrated considerable antibacterial efficacy. Notably, the scaffold containing VnNMs showed stronger antibacterial activity compared to the Alg-F scaffold, both against S. aureus (p < 0.05) and E. coli (p < 0.001). Stroescu et al.21 prepared chitosan-Vn films and demonstrated that the resulting films produced an inhibition zone of 11 ± 1.3 mm against E. coli after 24 h of incubation. Also, Ibrahim et al.34 developed a chitosan/polyvinyl alcohol/vanillin hydrogel loaded with L-arginine and reported that the fabricated hydrogel exhibited an inhibition zone of 31.32 ± 3.87 mm against S. aureus and 21.92 ± 5.21 mm against E. coli after 24 h of incubation. Therefore, these literature-reported inhibition zone values show a comparable trend to those observed in the current study.
(a) Images of the inhibition zones generated by different scaffolds against S. aureus and E. coli, (b) inhibition zone diagram, and (c) antibacterial activity of the scaffolds against S. aureus and E. coli.
The antibacterial effect of the Alg-F scaffold was solely attributed to the presence of F. The antibacterial properties of F are closely linked to glycoprotein receptors found on the surface of polysaccharides. These receptors exhibit a remarkable ability to bind with key bacterial structures—including the cell wall, cytoplasmic membrane, and DNA—ultimately disrupting essential bacterial functions and inhibiting growth35. It was revealed that F possess the ability to hinder the growth of both Gram-positive and Gram-negative bacteria, showcasing their potent and wide-ranging antibacterial efficacy36. On the other hand, the release of Vn was responsible for the stronger antibacterial activity of Alg-F-VnNMs scaffold compared to Alg-F scaffold. This plant-derived phenolic formaldehyde compound causes antibacterial activity by pore-forming and disrupting the integrity of the bacterial membrane, as well as inhibiting energy metabolism13,15. It has been demonstrated that Vn can act synergistically with other antibacterial agents, enhancing their effectiveness and leading to bacterial eradication12,14. Therefore, the enhanced antibacterial activity of the Alg-F-VnNMs scaffold compared to other scaffolds is entirely justified. In this study, antibacterial activity was assessed against two bacterial strains, S. aureus and E. coli, which are commonly involved in wound infections. However, to better understand the broader antibacterial potential in wound healing applications, further evaluation against a more diverse set of pathogenic strains is needed.
In vitro cytocompatibility evaluation
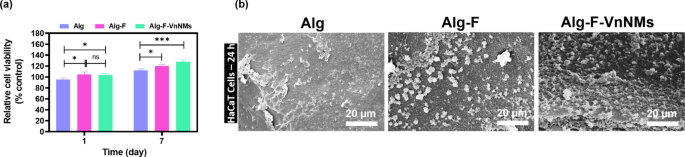
The HaCaT cells viability and their adhesion to the scaffold surfaces were evaluated as indicators of cytocompatibility. The MTT assay results, shown in Fig. 4a, demonstrate that all scaffolds exhibited appropriate cellular compatibility, with no scaffold-induced cytotoxicity observed. Overall, the Alg-F and Alg-F-VnNMs scaffolds showed higher levels of cell viability and proliferation compared to the Alg scaffold (p < 0.05). On day 1, there was no significant difference in cell viability between the Alg-F and Alg-F-VnNMs scaffolds (p > 0.05); however, after 7 days, cell viability on the Alg-F-VnNMs scaffold increased significantly compared to the Alg-F scaffold (p < 0.05).
(a) Viability assessment of HaCaT cells cultured on different scaffolds for 1 and 7 days, and (b) SEM images illustrating HaCaT cell adhesion on scaffolds after 24 h of culture.
Morphological analysis of cultured cells after 24 h on the scaffolds was performed using SEM imaging, with the results presented in Fig. 4b. Based on the findings, cells cultured on the scaffolds are observable at varying densities with almost spherical morphology. Weak cellular adhesion occurred on the Alg scaffold, which is consistent with other studies that have also reported poor cell adhesion on pure Alg surfaces37,38. SEM images indicate that cells adhered to the surfaces of Alg-F and Alg-F-VnNMs scaffolds with higher density and broader coverage. Interestingly, cellular expansion appeared to be more prominent on the Alg-F-VnNMs scaffold compared to the Alg-F scaffold.
The increase in surface hydrophilicity with the incorporation of F39 and the role of F as a preventive agent against cell apoptosis induced by oxidative stress40 were the two key factors contributing to the enhanced cellular behavior in the Alg-F scaffold compared to the Alg scaffold. According to Ryo et al.41, F mitigates oxidative stress in HaCaT cells by modulating the expression of heme oxygenase-1 (HO-1) and superoxide dismutase-1 (SOD-1). This regulation is mediated through the Nrf2/ERK signaling pathway, which plays a crucial role in cellular defense mechanisms against oxidative damage. Furthermore, it has been reported that Vn (in non-toxic concentrations) not only promotes stemness and self-renewal in HaCaT keratinocytes by regulating the expression of Oct-4, Nanog, and phosphorylated Oct-4 (p-Oct-4), thereby facilitating their regenerative capacity and long-term maintenance, but also enhances the expression of E-cadherin in HaCaT cells, allowing them to tightly integrate with surrounding cells and more effectively receive signals from their niches42. Thus, the improved HaCaT cell behavior observed on the Alg-F-VnNMs scaffold compared to other scaffolds was a predictable outcome, attributed to the controlled release of Vn from this scaffold.
Hemolysis and blood clotting time assays
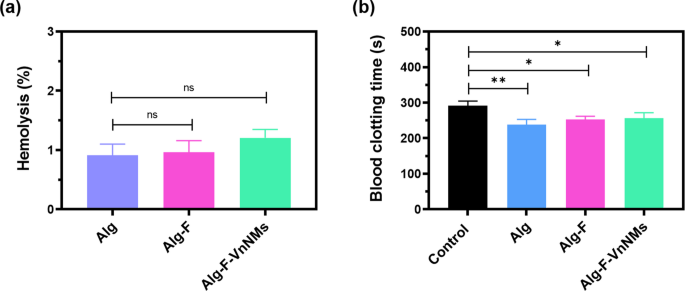
The hemolytic potential of the 3D-printed scaffolds was assessed to evaluate their compatibility with blood, and the corresponding data are presented in Fig. 5a. Hemolysis was quantified by measuring the amount of hemoglobin released, which reflects the extent of red blood cell membrane damage. According to established criteria, materials are generally considered blood-compatible if their hemolysis rate remains below 5%43, with some reports suggesting a stricter threshold of 2%26. In the present study, all scaffold samples exhibited hemolysis rates below 2%, indicating excellent hemocompatibility and classifying them as non-hemolytic.
Bar graph representation of blood–scaffold interaction assessments: (a) Hemolysis assay results reflecting the hemocompatibility of the fabricated scaffolds, and (b) Blood clotting time assay results.
As illustrated in Fig. 5b, the 3D-printed scaffolds demonstrated a notable enhancement in blood coagulation efficiency compared to the control group (sterile gauze). The clotting times recorded for the Alg, Alg-F, and Alg-F-VnNM scaffolds were 238.3 ± 14.7, 252.6 ± 9.0, and 256.6 ± 15 s, respectively, substantially shorter than the clotting time observed for control (291.3 ± 13.2 s). The reduced clotting time observed for the scaffolds relative to the control can be attributed to multiple factors, including the release of Ca2+ ions from calcium chloride used as a crosslinking agent during scaffold fabrication, which may promote coagulation upon blood contact, and the enhanced platelet adhesion supported by the scaffold’s high biocompatibility. Importantly, the reduction in clotting time was significantly greater for the Alg scaffold compared to the Alg-F and Alg-F-VnNMs scaffolds (p < 0.05), likely due to the presence of fucoidan in these scaffolds. Fucoidan contains sulfated moieties known to impart anticoagulant properties, thereby attenuating coagulation44.
In vivo animal studies
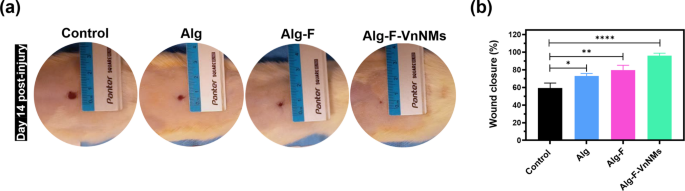
In vivo animal studies were conducted to evaluate full-thickness wound healing following the application of the fabricated scaffolds in a rat model over a 14-day period. On day 14 post-injury, the wound sites in different rats were photographed to assess the macroscopic appearance and quantify wound closure following the creation of circular excisional wounds with a diameter of 10 mm (Fig. 6a,b). All wounds demonstrated typical healing characteristics, with no visible abnormalities in color, texture, or surrounding tissue. Quantitative analysis of wound closure revealed a statistically significant enhancement in healing in the Alg and Alg-F groups compared to the control group (p < 0.05 and p < 0.01, respectively). Remarkably, the Alg-F-VnNMs group exhibited a pronounced pro-healing effect, outperforming all other groups. By day 14, approximately 95.8 ± 2.8% of the wound area had closed in this group, indicating a highly accelerated regenerative response.
(a) Representative macroscopic images of wound sites in rats on day 14 post-injury, illustrating the visual appearance of healing across experimental groups, and (b) quantitative analysis of wound closure percentage at day 14.
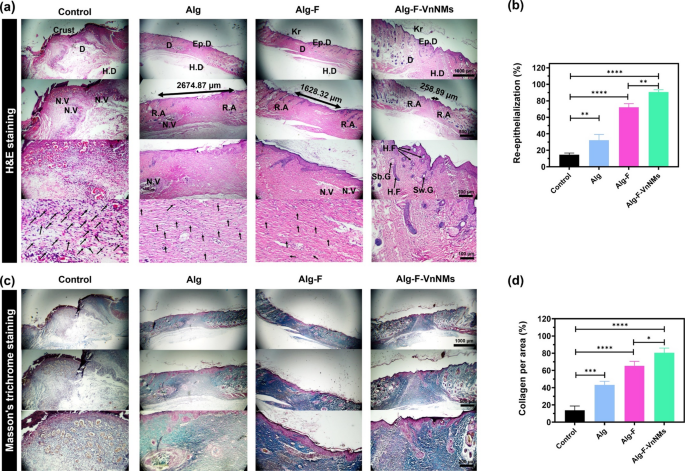
The histopathological results of the animal study are presented in Fig. 7. In Fig. 7a related to H&E-stained tissue sections, the essential histological attributes, including the keratin (Kr) layer, epidermis (Ep.D), dermis (D), hypodermis (H.D), regenerated area (R.A), neovascularization area (N.V), hair follicle (H.F), sebaceous gland (Sb.G) and sweat gland (Sw.G) are clearly marked in the images for each group. Also, inflammatory cells (If.C) were indicated by arrows. In the control group, Ep.D and Kr layers did not form after 14 days and are not visible in the images, and only a wound crust and large areas of N.V along with a lot of If.Cs are recognizable. In the group treated with the Alg scaffold, the Ep.D layer developed but lacked structural integrity, while the N.V regions were smaller and If.Cs were fewer compared to those in the control group. All three layers of H.D, D, and Ep.D were well formed in the groups treated with the Alg-F and Alg-F-VnNMs scaffolds, the N.V. areas and If.Cs were greatly reduced, and even H.Fs can be detected in the tissue slice images of these groups. However, the formation of an integrated and developed Kr layer, negligible number of If.Cs, and the presence of other skin appendages, including Sb.G and Sw.G, confirms the more complete wound healing of the Alg-F-VnNMs group compared to the Alg-F group after 14 days. A noteworthy point is that balancing angiogenesis and regression is essential for proper tissue repair. In the final stages of healing process, apoptosis in capillaries drives vascular regression, restoring density to normal levels. While early angiogenesis supports healing, delayed regression increases the risk of hypertrophic scars45. Therefore, the presence of extensive N.V areas in tissue sections of the control and Alg groups after 14 days indicates that the natural healing process has not been completed or that the healing has been delayed. In this study, the first H.F reaching the skin surface was considered as the R.A boundary, and the distance between the R.As on both sides of the wound actually indicates the unrepaired area. In the control group, there was no R.A with the mentioned features. The diameter of unrepaired area for the Alg, Alg-F, and Alg-F-VnNMs groups was measured to be 2674.87, 1628.32, and 258.89 µm, respectively, showing almost complete regeneration in the Alg-F-VnNMs group. Also, quantitative analysis of re-epithelialization based on H&E-stained tissue sections in Fig. 7b demonstrated a markedly improved epithelial coverage in all treatment groups compared to the control. The highest level of re-epithelialization was observed in the group treated with the Alg-F-VnNMs scaffold, showing a statistically significant enhancement relative to the control (p < 0.0001).
Histological evaluation and quantitative analysis of wound healing following a 14-day in vivo examination: (a) H&E-stained tissue slices (some inflammatory cells are marked with arrows), (b) quantitative analysis of re-epithelialization, (c) Masson’s trichrome-stained tissue slices, and (d) quantification of collagen content per area.
As depicted in Fig. 7c, the tissue sections of various groups stained with Masson’s trichrome reveal collagen deposition and maturation, distinctly visualized in blue. Collagen deposition in the control group lacks organization, whereas in the R.As of the Alg group, collagen bundles exhibit increased thickness compared to the control. Quantitative assessment of collagen deposition per area, based on Masson’s trichrome-stained sections, revealed significantly higher collagen accumulation in all treatment groups compared to the control (Fig. 7d). Collagen content in the Alg-F group was significantly greater than that in the Alg group (p < 0.05), while the Alg-F-VnNMs group exhibited even higher levels than Alg-F (p < 0.05). Accordingly, the Alg-F-VnNMs group demonstrated the most pronounced collagen synthesis among all experimental groups. As can be seen in the tissue sections stained with Masson’s trichrome, the N.V. regions in both the control and Alg treated groups appear distinctly visible. Although N.V regions remain visible in the Alg-F treated group, their presence is far less distinguishable in the Alg-F-VnNMs group. Also, the Alg-F and Alg-F-VnNMs groups exhibit a markedly greater density and more structured arrangement of collagen bundles compared to both the control and Alg groups. These findings indicate that the Alg-F-VnNMs group successfully achieved almost full wound healing within 14 days.
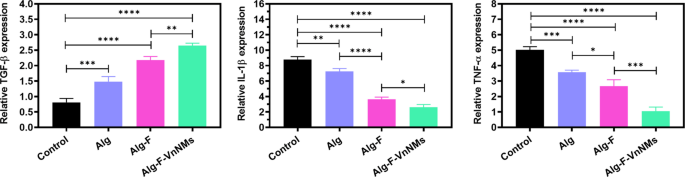
The expression of TGF-β, TNF-ɑ, and IL-1β genes in wound tissues were assessed via qRT-PCR and the results are presented in Fig. 8. The role of TGF-β in wound healing is essential, as it governs keratinocyte cell cycle regulation, facilitates re-epithelialization, and orchestrates key processes such as angiogenesis, inflammation, and granulation tissue development46. All treated groups had higher levels of TGF-β expression than the control group. The level of TGF-β expression in the Alg-F and Alg-F-VnNMs groups was higher than that in the Alg group, and the highest level of TGF-β expression was associated with the Alg-F-VnNMs scaffold-treated group. The expression of pro-inflammatory cytokines TNF-ɑ, and IL-1β was also significantly lower in the treated groups than in the control group. The Alg-F scaffold significantly reduced the expression of pro-inflammatory genes compared to the Alg scaffold, and the lowest expression of these pro-inflammatory genes was related to the group treated with the Alg-F-VnNMs scaffold.
Quantitative analysis of TGF-β, TNF-ɑ, and IL-1β genes’ expression at the wound tissues in different groups.
It is reported that F exhibits promising therapeutic potential for treating wounds and burns, as its heparin-like properties stimulate TGF-β. Additionally, it plays a crucial role in facilitating fibroblast migration and activation within injured tissue47. It has also been established that the anti-inflammatory mechanism of F is based on the downregulation of inflammatory mediators such as IL-1β, IL-6, IL-8, TNF-α, nitric oxide (NO), prostaglandin E2 (PGE2), inducible NO synthase (iNOS), and cyclooxygenase-2 (COX-2), while simultaneously upregulating interferon gamma (IFN-γ) and IL-10, thereby playing a crucial role in modulating inflammatory pathways48. On the other hand, studies have confirmed that Vn effectively suppresses pro-inflammatory cytokines such as IL-1β and TNF-α, while simultaneously enhancing the secretion of IL-4, IL-10, and TGF-β49. This dual action reinforces its anti-inflammatory properties and contributes to wound healing acceleration. de Aragão Tavares et al.50 demonstrated that the presence of Vn in a chitosan-based membrane played a pivotal role in modulating gene expression in diabetic wound tissues by suppressing TNF-α and IL-1β, while concurrently upregulating TGF-β, thereby fostering a more conducive environment for tissue regeneration.
These results highlight the effectiveness of the Alg-F-VnNMs scaffold in wound healing and skin tissue regeneration, demonstrating its superior performance compared to other scaffolds assessed within a 14-day evaluation period. This enhanced performance is attributed to the synergistic effect of the presence of F and Vn, which significantly contributed to the accelerated repair process.