Introduction
Pathophysiology of cancer cachexia
Clinical features and symptoms
Prevalence and clinical impact
Diagnosis and staging
Management strategies
Challenges and research directions
Conclusions
References
Further reading
Cancer cachexia is a multifactorial syndrome marked by muscle and fat loss, driven by inflammation and metabolic dysregulation beyond malnutrition. It worsens quality of life, limits treatment tolerance, and requires multimodal management strategies.
 Image Credit: Ground Picture / Shutterstock.com
Image Credit: Ground Picture / Shutterstock.com
Introduction
Cancer cachexia is a complex metabolic syndrome characterized by the ongoing loss of skeletal muscle mass, with or without fat loss, that cannot be reversed entirely by conventional nutritional support and results in progressive functional decline.1
Unlike simple weight loss or malnutrition, which are often reversible and primarily due to inadequate intake, cachexia involves involuntary and irreversible muscle wasting due to metabolic dysregulation and inflammation. It is underpinned by a sustained negative protein and energy balance driven by reduced intake and abnormal metabolism, distinguishing it clinically and biologically from starvation or isolated malnutrition.1,2
This article examines the causes and clinical manifestations of cancer cachexia, as well as current management strategies aimed at improving patient outcomes.
Pathophysiology of cancer cachexia
Complex cross-talk between tumor-derived factors and host responses leads to metabolic alterations such as increased autophagy, nutrient sequestration, proteolysis, and mitochondrial dysfunction. Key signaling nodes include JAK/STAT (particularly STAT3), NF-κB, and FOXO transcription factors that upregulate muscle ubiquitin ligases (MuRF1/MAFbx), while myostatin/activin A–SMAD2/3 signaling via ActRIIB suppresses anabolism. Cancer cachexia also disrupts hormonal mediators, such as insulin-like growth factor-1 (IGF-1), transforming growth factor-beta (TGF-β), leptin, and ghrelin, which are present in the liver, bones, adipose tissue, and stomach, respectively, thus highlighting the systemic effects of this condition.
Increased energy expenditure due to accelerated muscle and fat loss also contributes to symptoms of cachexia. Dysregulation in the ubiquitin-proteasome system, autophagy-lysosomal pathway, and calcium-dependent proteolytic processes leads to skeletal muscle wasting. Adipose tissue browning and lipolysis (e.g., PTHrP-driven thermogenesis) further amplify hypermetabolism, and mitochondrial uncoupling further impairs adenine triphosphate (ATP) production to promote hypermetabolism and inefficient energy use.3,5
Cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-1β stimulate protein breakdown, suppress protein synthesis, and induce anorexia through the hypothalamus. Dysregulation of hypothalamic appetite circuits (e.g., melanocortin system) contributes to anorexia and early satiety. Tumor-derived mediators, including proteolysis-inducing factor and parathyroid hormone-related protein (PTHrP), also amplify catabolism and disrupt appetite, contributing to systemic inflammation. 2,3
Cachexia suppresses anabolic signaling through the mechanistic target of rapamycin (mTOR) and IGF-1; however, catabolic pathways remain active. This imbalance results in negative nitrogen and energy balance, muscle atrophy, impaired function, reduced treatment tolerance, and higher mortality. Multi-organ effects (gut barrier dysfunction/microbiome changes, cardiac remodeling, and central neuroinflammation) further sustain the syndrome.3,4
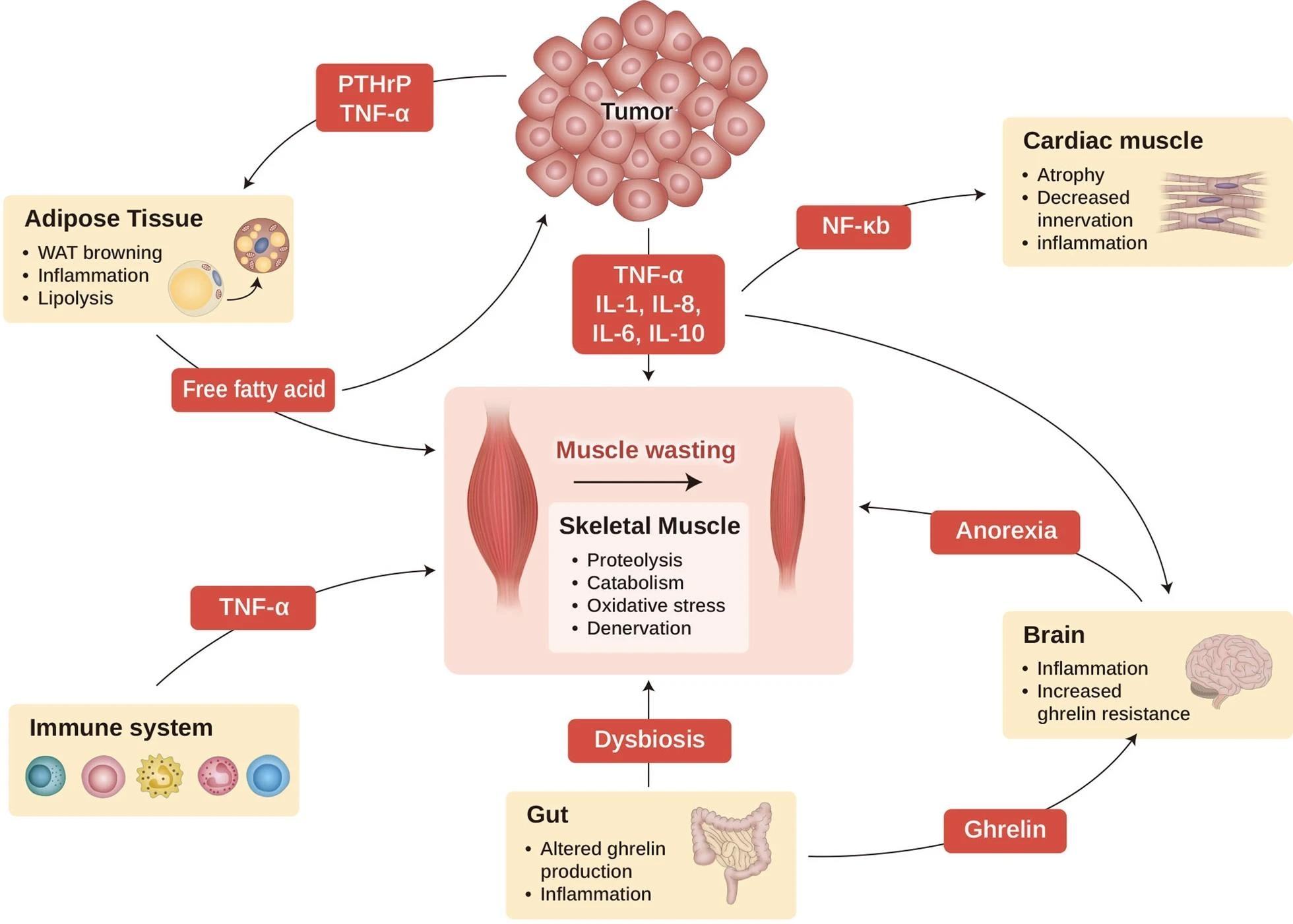
Cancer cachexia as a multi-organ syndrome. This scheme illustrates the interaction of major organs commonly associated with and affected by cachexia. Cancer cachexia that happens in the muscle (center) is dependent on the alterations in other organs, such as adipose tissue, brain, gut, cardiac muscle, and immune cells. Cachexia-inducing tumors secrete many factors, such as cytokines, PTHrP, and other mediators, to induce muscle wasting directly, as well as affecting other organs such as the brain, cardiac muscle, gut, and adipocyte tissue, which aggravates cachexia syndrome. WAT, white adipocyte tissue; PTHrP, parathyroid hormone-related protein; TNF-α, tumor necrosis factor-α; IL-1, interleukin-1; IL-6, interleukin 6; IL-8, interleukin-8; IL-10, interleukin 10; and NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells.3
Clinical features and symptoms
Cancer cachexia is characterized by ongoing weight loss, which is primarily due to skeletal muscle, fat, and, in some cases, cardiac muscle loss. Unlike malnutrition, these changes reflect profound metabolic disturbances that cannot revert to normalcy through nutrition.5
Muscle loss leads to weakness, fatigue, and lower physical performance. Patients often struggle with daily activities, which leads to more time spent in bed or seated, resulting in reduced mobility and independence. Reduced physical function also weakens social interactions and body image.2,3
Appetite loss is common, and many patients experience altered taste or difficulty digesting and absorbing nutrients. Early satiety, dysphagia (in head and neck or upper GI cancers), and dysgeusia are frequent contributors to intake reduction, and caregivers may respond by encouraging repeated feeding, which can cause nausea, vomiting, regurgitation, or aspiration pneumonia.5
Patients diagnosed with cancer cachexia may also experience emotional distress, frustration, and guilt over weight loss and poor appetite, whereas caregivers face emotional strain from multiple feeding challenges and complications. This dual burden intensifies the overall impact of this disease.4,5
Prevalence and clinical impact
Cancer cachexia affects up to 70% of patients with advanced malignancies and contributes to about 22% of cancer-related deaths. The prevalence of cancer cachexia varies by tumor type, with 80-87% of patients with pancreatic and gastric cancers diagnosed with this condition. Approximately 60% of patients are diagnosed with lung, colon, and prostate cancers, as well as non-Hodgkin lymphoma, whereas about 40% with breast cancer, sarcoma, leukemia, and Hodgkin lymphoma.5,6
Cancer cachexia also leads to fatigue, muscular atrophy, and psychological discomfort, all of which significantly reduce patients’ quality of life. This condition may also reduce tolerance to cancer therapies like chemotherapy, radiation, and surgery, which can lead to treatment delays, dose reductions, or dose interruptions, as well as an increased risk of infection and poor wound healing. These effects collectively worsen morbidity, mortality, and healthcare utilization.5
Cancer cachexia develops along a continuum, with pre-cachexia defined as ≤ 5% weight loss with anorexia and early metabolic changes, and cachexia is characterized by > 5% weight loss or sarcopenia with ongoing loss. Alternatively, diagnostic cut points include >2% weight loss with BMI <20 kg/m2, or sarcopenia with >2% loss, and refractory cachexia is often an advanced and treatment-resistant disease with poor performance status and a life expectancy of less than three months.1
The severity of cancer cachexia also varies by tumor type, extent of inflammation, and treatment response, with earlier onset and more pronounced progression observed in lung and pancreatic cancers. In advanced disease, cachexia is often irreversible, which is typically managed with palliative care.7,8
Diagnosis and staging
The Evans criteria define cachexia as 5% or more weight loss in less than 12 months in the presence of chronic disease. Reduced muscle strength, fatigue, anorexia, low fat-free mass index, or abnormal biochemistry characterized by increased C-reactive protein (CRP) and IL-6 levels, as well as anemia and low serum albumin.5
For cancer specifically, the 2011 international consensus (Fearon et al.) defines cachexia as: (i) >5% weight loss in the past 6 months (absent simple starvation), or (ii) BMI <20 kg/m2 with >2% weight loss, or (iii) sarcopenia with >2% weight loss, and recognizes a trajectory from pre-cachexia to cachexia to refractory cachexia.1 Other proposed clinical staging tools include CASCO/miniCASCO, the Cancer Cachexia Staging (CCS) system, and BMI-adjusted weight-loss grades to stratify severity and prognosis.4,7
The Patient-Generated Subjective Global Assessment (PG-SGA), Mini Nutritional Assessment (MNA), and Nutritional Risk Screening (NRS-2002) can be used to assess nutritional status. Cross-sectional imaging (e.g., CT at L3) is widely used to quantify skeletal muscle; DXA and BIA are alternative methods when CT is unavailable.
The characteristic weight loss of cancer cachexia can be ambiguous and resemble other conditions like malnutrition, sarcopenia, or anorexia. As compared to malnutrition, cachexia persists despite adequate nutrition.
Although anorexia may also be present, it does not account for the widespread inflammation, metabolic dysfunction, or persistent catabolism that occurs in cancer cachexia. Thus, cancer cachexia is a distinct and multifactorial syndrome that requires targeted interventions to preserve muscle mass, mitigate metabolic disruption, and improve functional outcomes.9,10
Management strategies
Cancer cachexia management is achieved through nutritional therapy that emphasizes high-calorie, high-protein diets, omega-3 fatty acids, and oral dietary supplements such as eicosapentaenoic acid, β-hydroxy-β-methylbutyrate, and L-carnitine. However, nutrition alone rarely reverses cachexia and is most effective when combined with other interventions.3,10 Early, structured nutrition pathways integrated into oncology care improve identification and support but do not substitute for anti-catabolic therapy.6
Appetite stimulants like megestrol acetate can improve appetite and weight gain but may increase the risk of thromboembolism, adrenal suppression, and fluid retention. Corticosteroids can also improve appetite and reduce the severity of common symptoms like nausea; however, these pharmaceutical agents cannot prevent muscle loss and are associated with numerous long-term effects such as osteoporosis, immunosuppression, and sleep disturbances that limit extended use.5
Anti-inflammatory agents, such as IL-6 antagonists (ALD518), IL-6R inhibitors (tocilizumab), and dual-target agents (OHR/AVR118), have been shown to stabilize body weight and reduce inflammation. Anamorelin, a ghrelin receptor agonist, increased lean body mass and body weight in advanced NSCLC (ROMANA 1/2) but did not improve handgrip strength; regulatory availability varies by region.11 In preclinical models, myostatin/activin A inhibitors, including soluble activin type IIB receptor (sActRIIB), preserved muscle integrity.5
Resistance and aerobic exercise support muscle maintenance, improve protein metabolism, and attenuate inflammation. Resistance training enhances strength and mobility, whereas aerobic exercise reduces muscle atrophy through the activation of adiponectin signaling.
Previously, a randomized controlled trial reported improvements in elbow and knee flexor/extensor muscles among pancreatic cancer patients after resistance training. Exercise improved functional independence; however, weight changes were modest.12
A multimodal approach combining nutrition, drugs, and exercise offers the best outcomes in cancer cachexia. These management strategies can preserve muscle, boost appetite, reduce inflammation, and improve quality of life, with psychosocial and supportive care further enhancing these benefits. Where feasible, early referral to rehabilitation, symptom control (pain, nausea), and management of tumor- or treatment-related contributors (e.g., dysphagia) should be embedded in care plans.5,10
Challenges and research directions
Cancer cachexia management remains challenging due to the limited efficacy associated with single interventions. Thus, there remains an urgent need for multimodal strategies that address both metabolic dysregulation and chronic inflammation.13
Although organizations such as the American Society of Clinical Oncology (ASCO) provide recommendations, there remains a lack of standardized treatment guidelines. Implementation of existing guidance (e.g., ESPEN/ASCO) into routine oncology pathways is suboptimal, and consistent screening plus early-stage intervention are key gaps.6,13
Hormonal and metabolic agents, such as selective androgen receptor modulators (SARMs) and β2-adrenergic agonists, like enobosarm and formoterol, have also been widely studied for their potential role in managing cancer cachexia. These therapies stimulate appetite, preserve lean mass, and improve function. However, confirmatory phase 3 evidence demonstrating durable functional benefit remains limited.
Immunomodulatory approaches, including IL-6 antagonists, target inflammatory and catabolic pathways central to muscle wasting. Numerous preclinical studies have also confirmed the anti-inflammatory effects of natural compounds like resveratrol, curcumin, and ginsenoside derivatives; however, safety concerns remain with agents like cannabinoids and testosterone. Future trials should prioritize patient-centred endpoints (function, treatment tolerance, survival) and standardized definitions/staging to enable comparison across studies.3,14
Conclusions
Cancer cachexia is a complex and multifactorial syndrome that affects metabolic, immune, and neurological systems. Optimal management requires a multidisciplinary approach that combines nutritional support, pharmacologic therapy, and exercise to preserve muscle mass and physical function.
Future research is needed to develop targeted anti-cachexia agents that enhance muscle growth, improve functional outcomes, and maintain quality of life. Understanding underlying mechanisms and identifying novel therapeutic targets will be pivotal for reducing morbidity, improving treatment tolerance, and ultimately extending survival in patients with cancer cachexia.
References
Fearon, K., Stasser, F., Anker, S. D., et al. (2011). Definition and classification of cancer cachexia: an international consensus. The Lancet Oncology 12(5); 489-495. DOI:10.1016/S1470-2045(10)70218-7, https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(10)70218-7/
Donohoe, C. L., Ryan, A. M., & Reynolds, J. V. (2011). Cancer Cachexia: Mechanisms and Clinical Implications. Gastroenterology Research and Practice, 601434. DOI:10.1155/2011/601434, https://onlinelibrary.wiley.com/doi/10.1155/2011/601434
Setiawan, T., Sari, I. N., Wijaya, Y. T., et al. (2023). Cancer cachexia: molecular mechanisms and treatment strategies. Journal of Hematology & Oncology 16; 54. DOI:10.1186/s13045-023-01454-0, https://jhoonline.biomedcentral.com/articles/10.1186/s13045-023-01454-0
Yue, M., Qin, Z., Hu, L., & Ji, H. (2024). Understanding cachexia and its impact on lung cancer and beyond. Chinese Medical Journal Pulmonary and Critical Care Medicine 2(2); 95-105. DOI:10.1016/j.pccm.2024.02.003, https://www.sciencedirect.com/science/article/pii/S2772558824000045
Ni, J., & Zhang, L. (2020). Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Management and Research 12, 5597. DOI:10.2147/CMAR.S261585, https://www.dovepress.com/cancer-cachexia-definition-staging-and-emerging-treatments-peer-reviewed-fulltext-article-CMAR
Sadeghi, M., Keshavarz-Fathi, M., Baracos, V., et al. (2018). Cancer cachexia: Diagnosis, assessment, and treatment. Critical Reviews in Oncology/Hematology 127; 91-104. DOI:10.1016/j.critrevonc.2018.05.006, https://www.sciencedirect.com/science/article/pii/S1040842817302731
Mariean, C. R., Tiucă, O. M., Mariean, A., & Cotoi, O. S. (2023). Cancer Cachexia: New Insights and Future Directions. Cancers 15(23), 5590. DOI:10.3390/cancers15235590, https://www.mdpi.com/2072-6694/15/23/5590
Hariyanto, T. I., & Kurniawan, A. (2020). Cachexia in Cancer Patients: Systematic Literature Review. Asian Journal of Oncology 6(3);107-115. DOI:10.1055/s-0040-1713701, https://asjo.in/cachexia-in-cancer-patients-systematic-literature-review/
Santos, J. M., & Medeiros, R. (2020). Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia, and asthenia. Journal of Cachexia, Sarcopenia and Muscle 11(3); 619. DOI:10.1002/jcsm.12528, https://onlinelibrary.wiley.com/doi/10.1002/jcsm.12528
Muthanandam, S., & Muthu, J. (2021). Understanding Cachexia in Head and Neck Cancer. Asia-Pacific Journal of Oncology Nursing 8(5); 527. DOI:10.4103/apjon.apjon-2145, https://apjon.org/article/S2347-5625(21)00079-2/fulltext
Temel, J. S., Abernethy, A. P., Currow, D. C., et al. (2016). Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncology 17(4);519–531. DOI:10.1016/S1470-2045(15)00558-6, https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(15)00558-6/abstract
Wiskemann, J., Clauss, D., Tjaden, C., et al. (2019). Progressive resistance training to impact physical fitness and body weight in pancreatic cancer patients: a randomized controlled trial. Pancreas 48(2):257-266. DOI:10.1097/MPA.0000000000001221, https://journals.lww.com/pancreasjournal/abstract/2019/02000/progressive_resistance_training_to_impact_physical.18.aspx
Arends, J., Musaritoli, M., Anker, S., et al. (2023). Overcoming barriers to timely recognition and treatment of cancer cachexia: Sharing Progress in Cancer Care Task Force Position Paper and Call to Action. Critical Reviews in Oncology/Hematology 185, 103965. DOI:10.1016/j.critrevonc.2023.103965, https://www.sciencedirect.com/science/article/pii/S1040842823000537
Wijaya, Y. T., Setiawan, T., Sari, I. N., et al. (2022). Ginsenoside Rd ameliorates muscle wasting by suppressing the signal transducer and activator of transcription 3 pathway. J Cachexia Sarcopenia Muscle 13(6):3149-62. DOI:10.1002/jcsm.13084, https://onlinelibrary.wiley.com/doi/10.1002/jcsm.13084
Further Reading
Last Updated: Sep 9, 2025