Experimental conditions and equipment
This study was approved by the Institutional Animal Care and Use Committee of Shihezi University (Approval No. A2024-392). All surgical procedures were performed in the Animal Laboratory of Shihezi University Animal Center, with strict adherence to aseptic techniques and animal welfare guidelines. Major equipment utilized included: Micro-CT scanner (Model NMC-200, PingSheng Medical Technology, China); Data acquisition software (Cruiser, PingSheng Medical Technology, China); Image reconstruction software (Recon, PingSheng Medical Technology, China); Surgical microscope (Model XT-X-4B, XinTian Medical Device Co., Ltd, China); Multi-parameter monitoring system (Vetal 3, Mindray, China); Medical oxygen concentrator (Model ROC-5 A, RWD, China).
Experimental animals
Species
Adult New Zealand White rabbits.
Quantity
48 male subjects.
Weight Range
2.0–2.5 kg (mean 2.25 ± 0.15 kg).
Source
Shihezi University Laboratory Animal Center Certification No.: SYXK(New)2023-0003.
Experimental reagents and surgical instruments
Pharmacological Agents:
Anesthetics
Propofol (Veterinary use, 10 ml:100 mg; Jiabo Pharma, Guangdong, China); Xylazine HCl (Veterinary injection, 2 ml:0.2 g; FangZheng Animal Pharma, Jilin, China).
Antimicrobials
Ceftiofur Sodium (20 ml:2 g; AMICOGEN, Shandong, China); Erythromycin Ointment (1% w/w, 10 g; KeRui Pharma, Chongqing, China).
Supportive Medications
0.9% Sodium Chloride Injection (500 ml/bag; Likang Pharma, Sichuan, China).
Surgical Instruments
Laminectomy Rongeur (2 mm cutting width; Cr P2F035; JingChuang Medical, Shanghai); Anti-needlestick IV Catheter (24G×0.7 mm, 20 ml/min flow rate).
Support Equipment
Infusion Pump (Model CP-660TC; SiLuGao Medical, Beijing); Surgical LED Light (Model ZS-LED500; ZhongShiDiChuang, Beijing).
Animal model establishment protocolPreoperative evaluation
A comprehensive anesthetic evaluation was performed on the experimental rabbits, including assessments of body weight, general nutritional status, and responsiveness to external stimuli, to ensure the effectiveness and safety of the anesthesia process.
Anesthesia protocol
(1)
First, the experimental rabbits were fixed in a dedicated experimental box. The ear marginal vein was located, and local skin preparation with alcohol disinfection was performed to fully expose the vein. A 24G yellow indwelling needle was used for ear marginal vein puncture and catheterization. After successful establishment of the ear marginal venous access, 3 mg/kg of veterinary 1% propofol was slowly injected intravenously to induce anesthesia. Once induction anesthesia was successful, the rabbits were fixed in a prone position on the animal experiment table. A micro-infusion pump was connected to continuously infuse 3 mg/kg of 1% propofol through the ear marginal vein at a rate of 150 mg/h. Meanwhile, 1.5 mg/kg of veterinary xylazine injection was slowly injected intravenously for auxiliary analgesia, with additional doses administered intraoperatively based on the rabbits’ pain responses. It was ensured that the rabbits remained in an anesthetized state throughout the surgery, without spontaneous movement or episodes of excitement and restlessness.
(2)
After induced anesthesia and throughout the surgical procedure, the experimental rabbits were provided with oxygen support using an oxygen generator (ROC-5 A, RWD, China) at an oxygen flow rate of 1–1.5 L/min. A benchtop multi-parameter monitor (Vetal 3, Mindray, China) was used for continuous monitoring, and their vital signs were closely monitored and recorded. A constant-temperature operating table set at 37 °C was used for maintaining body temperature to ensure the safe conduct of the surgery.
Surgical procedure
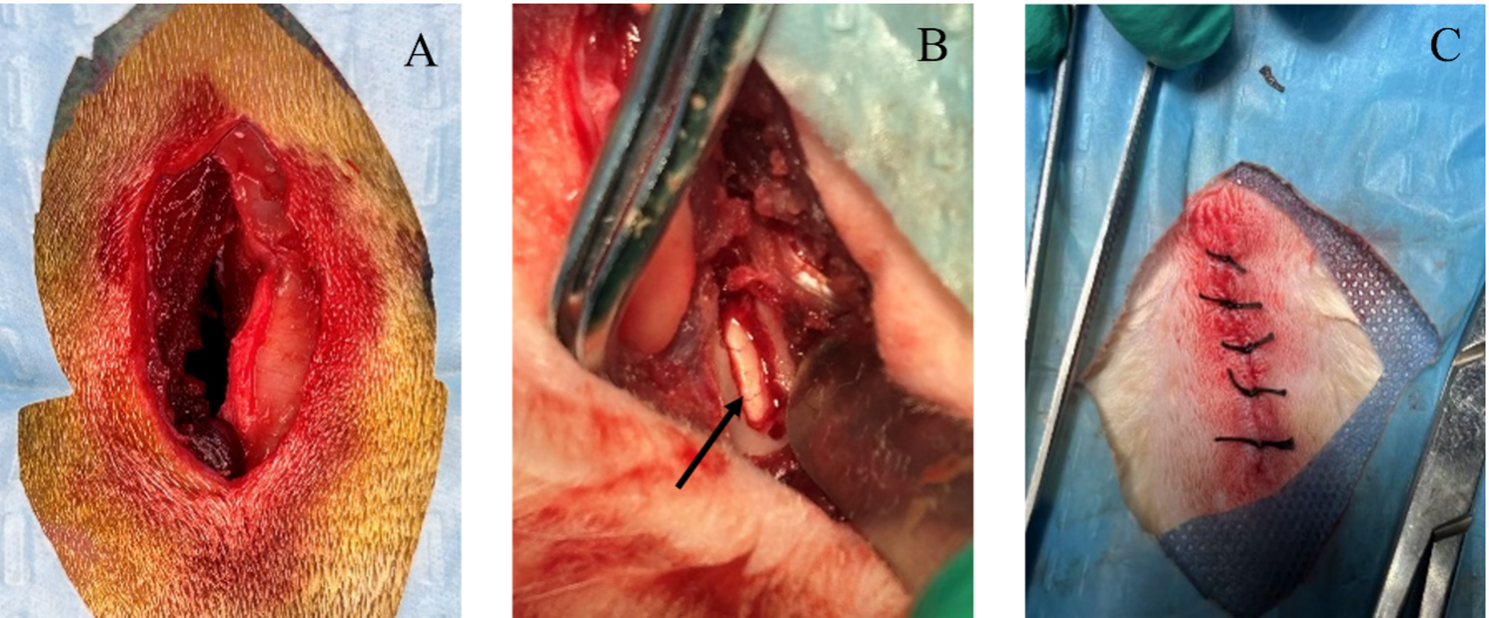
Following successful induction of general anesthesia and analgesia, anatomical localization was achieved by palpating the iliac crest as the primary osseous landmark to identify the L5-L6 interlaminar space. The local area was prepared using an animal hair clipper, followed by standard disinfection three times and draping with 2 layers of sterile surgical towels. A longitudinal incision approximately 4 cm in length was made parallel to the long axis of the L5-6 spinous processes, centered between the L5-6 spinous processes. The skin, fascial layer, muscular fascial layer, and the muscle layer adjacent to the left edge of the L5-6 spinous processes were incised sequentially. The local muscle tissue was bluntly dissected to expose the unilateral laminar space. A 2.0 mm laminectomy forceps was used to remove parts of the upper and lower laminae and the ligamentum flavum, allowing clear visualization of the white dura mater and its distributed blood vessels. At the end of the surgery, the surgical site was irrigated with normal saline three times. The incision was sutured layer by layer, including the muscular fascial layer, fascial layer, and skin layer. After suturing, the skin was disinfected with iodophor, an appropriate amount of erythromycin ointment was applied, and the incision was dressed (Fig. 1).
Surgical images. (A) Surgical incision; (B) Black arrow indicates: microscopic laminotomy; (C) Surgical suture
Postoperative management
Animals were allowed to recover spontaneously in a temperature-controlled recovery chamber (20 ± 2 °C). Postoperatively, intramuscular injection of ceftiofur (30 mg/kg) in the gluteal region for anti-infection and subcutaneous injection of meloxicam (1 mg/kg) [25] were administered daily for 3 consecutive days. Additionally, close observation was conducted on the rabbits’ pain conditions, mental status, dietary intake, incision healing, and lower limb mobility, with timely detection and management of any potential complications.
Monitoring indicators
The anesthetic state was evaluated using the definition of the anesthetic states and attributed numerical scale [21] (Supplementary Materials 1). Preoperative and intraoperative oxygen saturation (%), respiratory rate (breaths/min), body temperature (°C), and heart rate (beats/min) were monitored to ensure stable vital signs. Intraoperative blood loss (ml), surgical duration (min), anesthesia duration (min), and postoperative awakening time (min) were assessed and recorded, and a learning curve for surgical duration was plotted.
Methods for model validation
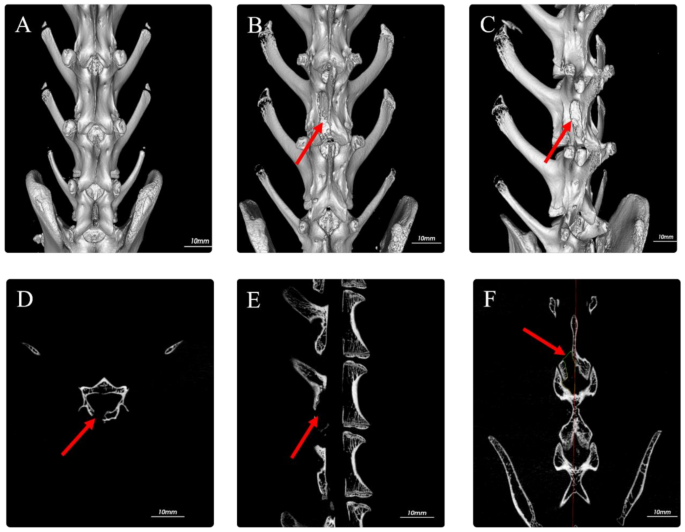
Intraoperative microscopic observation: The surgical procedure was considered successful if parts of the lamina were resected, the ligamentum flavum was removed, and the dural sac and nerve roots were fully exposed. Postoperatively, the lower limb mobility and incision healing were observed. Preoperatively and 1 day postoperatively, the experimental rabbits underwent lumbar Micro-CT scans, and the area of the laminar window was measured in the coronal plane to further verify the success of the fenestration (Fig. 2).
Micro-CT images. (A) Preoperative Micro-CT 3D; (B) Postoperative Micro-CT 3D; (C) Postoperative Micro-CT 3D rotated 45°; (D) Postoperative axial view; (E) Postoperative sagittal view; (F) Postoperative coronal view showing laminotomy area measurement range (red arrow indicates: L5-6 laminotomy site)
Statistical methods
Data processing and graphing were performed using SPSS (v27.0, USA) and R statistical analysis software. Normality tests were conducted for continuous data. For data that conformed to a normal distribution with homogeneous variance, the t-test was used for analysis; for data that did not conform to a normal distribution or with heterogeneous variance, the Mann-Whitney U test was applied, and the results were expressed as median (interquartile range). For continuously measured data, two-way repeated measures analysis of variance was used, and the results were presented as mean ± standard deviation. Categorical data were expressed as counts and rates (%), and the chi-square (χ²) test was used for comparison. A P value < 0.05 was considered statistically significant.