Implantable Medical Devices Market
Segmentation Analysis:
By Product Analysis:
The cardiovascular implants segment
dominates in the implantable medical devices market, as these types of implants
find broad medical healthcare applications, making an excessive contribution to
the management of cardiovascular diseases. Increasing incidence of chronic and
cardiovascular diseases (CVDs), and technological advancement, with limited
biological replacement options available. These devices are related to lawful
implications, including malpractice lawsuits associated with negligence and
product liability. It supports the heart in maintaining a steady and steady
heart rhythm and flow of blood in the body.
On the other hand, the dental implants
segment is expected to grow significantly during the forecast period, as they
are permanently attached to the jaw, and they offer enhanced oral comfort
compared to dentures, which slip and move in the mouth. Dental implants offer a
natural-feeling and natural-looking replacement for a lost tooth that lasts for
many years. It supports preserving speech patterns by filling holes in teeth
that cause variations in the way air passes by the mouth when talking.
By Biomaterial Analysis:
The metallic segment dominated the market
in 2024, as this type of implant is the primary biomaterial used for joint
replacement and is becoming progressively important. The metallic implants used
for orthopedic applications are categorized as CoCr alloys, stainless steel,
and Ti and Ti alloys. These metallic materials have numerous characteristics
such as high strength, high fracture toughness, corrosion resistance, hardness,
and biocompatibility, which make them an outstanding choice for entire joint
replacement.
On the other hand, the natural segment is
expected to grow at the fastest CAGR in the market during the forecast period,
as it provides advantages such as a natural entrance and durability, feel,
enhanced function, and chronic oral health advantages. They are attached to the
jawbone, offering stability for eating and speaking, and last a lifetime with
appropriate care. Implants are intended to look, function, and feel such as
natural teeth, supporting to restoration of confidence.
By End Use Analysis:
The hospitals segment dominated the market
in 2024, as these implants are used to replace body parts such as hips or
knees, deliver medication such as for pain relief, monitor and regulate body
functions such as heart rate, and provide support to organs and tissues. Some
implants are inert and intended to provide structural support, such as medical
stents or meshes.
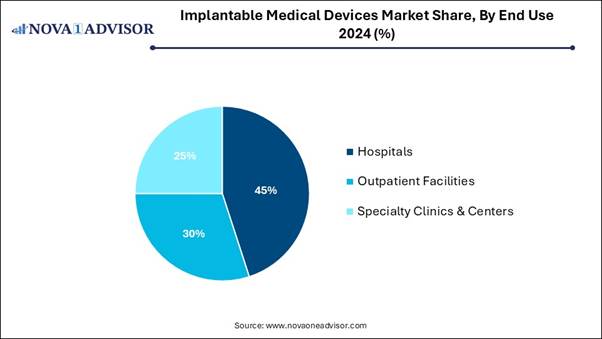
On the other hand, the outpatient
facilities segment is expected to grow at the fastest CAGR in the market during
the forecast period, as the most significant advantages of outpatient joint
replacement are the improved recovery time compared to outdated inpatient
surgeries. Outpatient processes often use Enhanced Recovery After Surgery
(ERAS) protocols, which focus on lowering the body’s strain response to
surgery. Outpatient joint replacement predominantly lowers the economic burden
on patients and the medical care system.
By Regional Insight
North America dominated the implantable
medical devices market in 2024, due to its strong and well-developed medical
care infrastructure, including leading specialized treatment centers and
hospitals. These services are equipped with modern technology for multifaceted
clinical procedures and medical care. Increasing incidence of chronic diseases,
such as cardiovascular and nervous system disorders, which are major driver of
demand for implants such as neurostimulators, stents, and pacemakers.
🔹For Instance, In July 2025, Inari Medical, now part of Stryker, a
global leader in medical technologies, announced the launch of its
next-generation InThrill Thrombectomy System, the first and only purpose-built
small vessel and arteriovenous (AV) access thrombectomy system that can deliver
fast, full luminal clot removal.
In the U.S. growing aging population is
more prone to age-associated health conditions, such as joint degeneration and
cardiovascular issues, further driving the requirement for implantable devices.
A rising preference for less invasive surgical procedures increases demand for
progressive implantable devices that provide rapid recovery times and lower
complications.
🔹For Instance, In March 2025, Boston Scientific Corporation announced
entered into a definitive agreement to acquire SoniVie Ltd., a privately held
medical device company that has developed the TIVUS Intravascular Ultrasound
System. An investigational technology, the TIVUS system is designed to
denervate nerves surrounding blood vessels to treat a variety of hypertensive
disorders, including renal artery denervation (RDN) for hypertension.
Why is Asia Pacific the Fastest Growing
in the Implantable Medical Devices Market?
APAC is the fastest-growing region in the
market, as the growing burden of chronic conditions like CVD disease, diabetes,
and cancer is increasing in the region, driven by urbanization and lifestyle
variations. This increases demand for healthcare technologies such as
orthopedic implants and cardiac stents. The acceptance of technologies like
artificial intelligence (AI), 3D printing, and smart implants is fast-tracking
in the region. Increasing government support in countries like India and China
is implementing favorable policies, including investment incentives for local
manufacturers, to lower import dependency and improve affordability.
🔹For Instance, In November 2024, the Indian government’s newly
launched Rs 500 crore MedTech scheme is expected to revitalise domestic
manufacturing within the medical equipment sector, which has largely shifted
towards importing and pseudo-manufacturing. The scheme, announced, seeks to
enable Indian manufacturers to become global exporters of medical devices and
respond to industry challenges, including skill shortages.
Implantable Medical Devices Market
Companies:
• Abbott Laboratories
• Biotronik SE and Co. KG
• Boston Scientific Corporation
• Cochlear Limited
• Institut Straumann AG
• Integra LifeSciences Corporation
• Johnson and Johnson
• LivaNova PLC
• Medtronic
• Smith & Nephew plc
• Stryker
What is Going Around the Globe?
⬥︎In October 2025, Medtronic plc.
announced the start of the Embrace Gynecology investigational device exemption
(IDE) U.S. clinical study to evaluate the safety and effectiveness of its Hugo
robotic-assisted surgery (RAS) system in robotic-assisted gynecological
procedures.
⬥︎In September 2025, Medtronic plc, a
global leader in healthcare technology, announced that it had received U.S.
Food and Drug Administration (FDA) approval for the Altaviva device. The
minimally invasive implantable tibial neuromodulation (ITNM) therapy is
inserted near the ankle and designed to treat urge urinary incontinence.
⬥︎In September 2025, Biogen Inc.
announced the company had entered into a definitive agreement to acquire
Massachusetts-based Alcyone Therapeutics. As part of an existing partnership
with Alcyone Therapeutics, the companies are advancing ThecaFlex DRx, an
implantable subcutaneous port and catheter device being investigated for the
intrathecal delivery of antisense oligonucleotides (ASOs).
⬥︎In October 2025, AtaCor Medical, Inc.,
a privately-held medical device company focused on transforming cardiac rhythm
management systems, announced today that it has entered into a financing of up
to $75 million. The proceeds will fund the company’s U.S. FDA Pivotal Study
evaluating AtaCor’s parasternal extravascular implantable
cardioverter-defibrillator (EV-ICD) system for the treatment of life-threatening
ventricular tachyarrhythmias.
You can place an order or ask any
questions, please feel free to contact at sales@novaoneadvisor.com |
+1 804 441 9344
Related Report –
🔹
Hi-Tech Medical Devices Market- https://www.novaoneadvisor.com/report/hi-tech-medical-devices-market
🔹
Nanotechnology in Medical Devices Market- https://www.novaoneadvisor.com/report/nanotechnology-in-medical-devices-market
🔹
Wearable Medical Devices Market- https://www.novaoneadvisor.com/report/wearable-medical-devices-market
🔹
U.S. Reprocessed Medical Devices Market- https://www.novaoneadvisor.com/report/us-reprocessed-medical-devices-market
🔹
Medical Devices Vigilance Market- https://www.novaoneadvisor.com/report/global-medical-devices-vigilance-market
🔹
U.S. Wearable Medical Devices Market- https://www.novaoneadvisor.com/report/us-wearable-medical-devices-market
Implantable Medical Devices Market
Segmentation:
Segments Covered in the Report
By Product
• Aesthetic Implants
• Cardiovascular Implants
• Dental Implants
• Neurology Implants
• Orthopedic Implants
• Ophthalmology Implants
By Biomaterial
• Ceramic
• Metallic
• Natural
• Polymers
By End Use
• Hospitals
• Outpatient Facilities
• Specialty Clinics & Centers
By Regional
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East and Africa (MEA)
Immediate Delivery Available | Buy This
Premium Research https://www.novaoneadvisor.com/report/checkout/9181
About-Us
Nova One Advisor is a global leader
in market intelligence and strategic consulting, committed to delivering deep,
data-driven insights that power innovation and transformation across
industries. With a sharp focus on the evolving landscape of life sciences, we
specialize in navigating the complexities of cell and gene therapy, drug
development, and the oncology market, enabling our clients to lead in some of
the most revolutionary and high-impact areas of healthcare.
Our expertise spans the entire biotech
and pharmaceutical value chain, empowering startups, global enterprises,
investors, and research institutions that are pioneering the next generation of
therapies in regenerative medicine, oncology, and precision medicine.
Web: https://www.novaoneadvisor.com/
Contact Us
USA: +1 804 420 9370
Email: sales@novaoneadvisor.com
For Latest Update
Follow Us: LinkedIn

