Signalment/history
A one-day-old, 35 kg, female black Aberdeen Angus calf was admitted to the Clinic for Cattle, University of Veterinary Medicine, Hannover, because of inability to stand. The dam was reared in Estonia and imported to Germany for superovulation and embryo transfer. Artificial insemination with Aberdeen Angus semen imported from the USA was done in Germany. An experienced veterinarian transferred the embryos to six different crossbreed heifers following the German Cattle Breeders Association protocol [18]; only one embryo transfer resulted in pregnancy. The calf was given 1 L of colostrum from a pool of frozen colostrum, obtained from a dairy farm, in the first 24 h after birth.
This case was examined by clinical signs, neurological tests, blood analysis, radiography and magnetic resonance imaging, and postmortem examination. Additionally, the blood specimen was obtained for cytogenetic analysing and whole genome sequencing.
Clinical findings
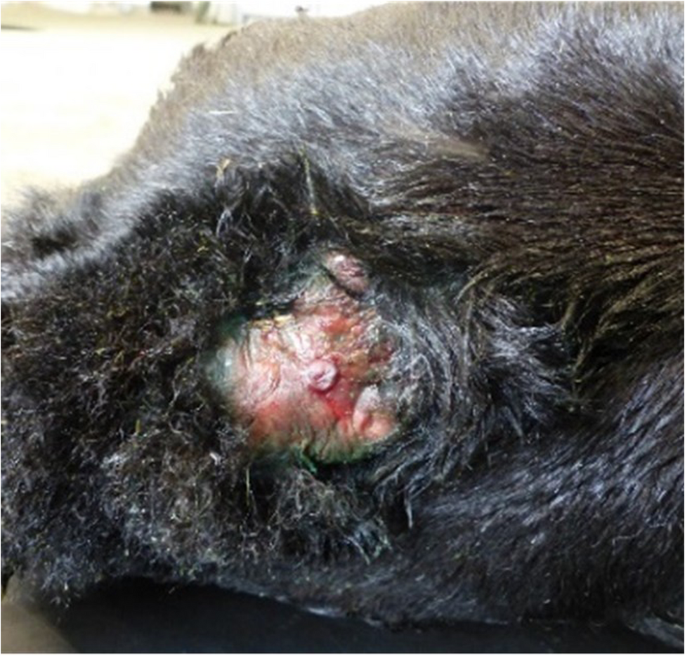
At admission to the clinic, the calf was alert and in sternal recumbency with the hind limbs extended to one side and the head positioned normally. The calf could not stand even with assistance, or fully extend both carpal joints, although both front limbs could be extended passively. The calf showed motivation to stand up but could only move the front limbs and was not able to rise on its own. A 6 cm wide, circular, pink excrescence with several wrinkles near to its border, which was well demarcated from the normal skin, was present over the caudal sacral region. In the centre of the hairless area, a raised papillary lesion with a central indentation, approximately 0.8 cm in diameter, was visible (Fig. 1). A probe inserted into the fistula-like area could not be extended into the deeper layers. Outflow of exudate or transudate from the excrescence was not observed via the hairless part. The calf was able to suckle milk from a nipple-bottle and remained alert during hospitalisation.
A one-day-old Aberdeen Angus calf with a raised hairless area and epidermal ulceration in the dorsal midline of the sacrum
Neurological examination
Neurological examination revealed normal spinal reflexes in the front limbs. The calf could move the front limbs but could not fully extent both carpal joints. In the hind limbs, paraparesis with decreased muscle tone and severe muscle atrophy was found. The patellar reflex was normal bilaterally, and the flexor reflex response was reduced to absent. The calf remained unable to stand despite daily assistance. The cutaneous trunci reflex was normal bilaterally up to the caudal lumbar area, where no response was elicited. General cutaneous sensitivity was normal in all limbs. Nevertheless, muscle fasciculations were observed in the dorsal skin regions when the hind limbs were stimulated. The tail was flaccid with no active movement. The anal sphincter and tonus were normal. The pupillary light reflex, facial nerve function, palate reflex, and lip tone were normal. The auricular reflex appeared delayed bilaterally; hearing was normal. On the first day postnatum, the calf did not react to visual stimuli. However, in the following days it was noted that visus was not sustained. The animal showed a ventral strabismus in the left eye, ventrolateral strabismus in the right eye, and mild bilateral exophthalmos.
Treatment
The animal was treated with iron(III)-hydroxide dextran complex (Belfer® 100 mg/ml, Bela-Pharm GmBH & Co. KG, Vechta, Germany, 0.1–0.3 ml/kg BW, s.c.), vitamins A, D, E and selenium (Ursovit®AD3EC, 30 mg/ml retinyl palmitate, 0.125 mg/ml cholecalciferol, 30 mg/ml alpha-tocopherol acetate, and 100 mg/ml ascorbic acid Serumwerk Bernburg AG, Bernburg, Germany, 5 ml/animal, s.c.), and vitamin E with selenium (Vitamin-E-Selen® ad us vet, 150 mg/ml all-rac-alpha-tocopherol acetate and 1.1 mg/ml sodium selenite, aniMedica GmbH, Senden-Bösensell, Germany, 5 ml/animal supplements).
Blood analysis
The total leukocyte count (31,900/µl [reference interval 8,000–10,000/µl] [19]), segmented neutrophils (85.0% [22.0–45.0%] [19]), total erythrocyte count (10.4*106/µl [6.0–8.0 *106/µl] [19]), and haematocrit (37.0% [25.0–35.0%] [19]) were increased. Lymphocytes were decreased (13.0% [45.0–65.0%] [19]). Liver variables, including parameters such as total bilirubin concentration (19.6 µmol/l [< 7.0 µmol/l] [19]) and the activity of gamma glutamyl transferase (212 U/l [< 33U/I] [19]) were increased. The concentration of cholesterol (1.43 mmol/l [>3.0 mmol/l] [19]), total protein (49 g/l [60.0–80.0 g/l] [19]) and albumin (25.3 mmol/l [30.0–40.0 mmol/l] [19]) were decreased. A serum ELISA for antibodies against infectious bovine rhinotracheitis virus (IBRV) was negative. Antigen ELISA and serum ELISA testing for BVDV antibodies were negative. Serum ELISA for antibodies was negative for BTV but positive for SBV.
Radiographic findings
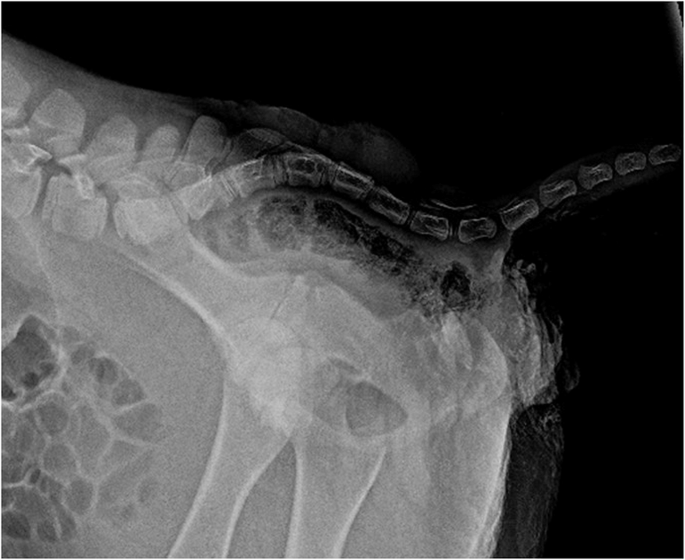
Laterolateral and ventrodorsal radiographic of the lumbosacral region were obtained without sedation (Amadeo P-125/100 VB, 5.6 kW, Oehm and Rehbein GmbH Rostock, Germany). The laterolateral view showed that the alignment of the lumbar and sacral spine was dorso-ventrally S-shaped (kyphosis) and absence of the bony dorsal arch in the caudal sacral part of the spinal canal (Fig. 2).
Radiograph showing the S-shaped spinal cord (kyphosis) in a one-day-old Aberdeen Angus calf
MRI Findings
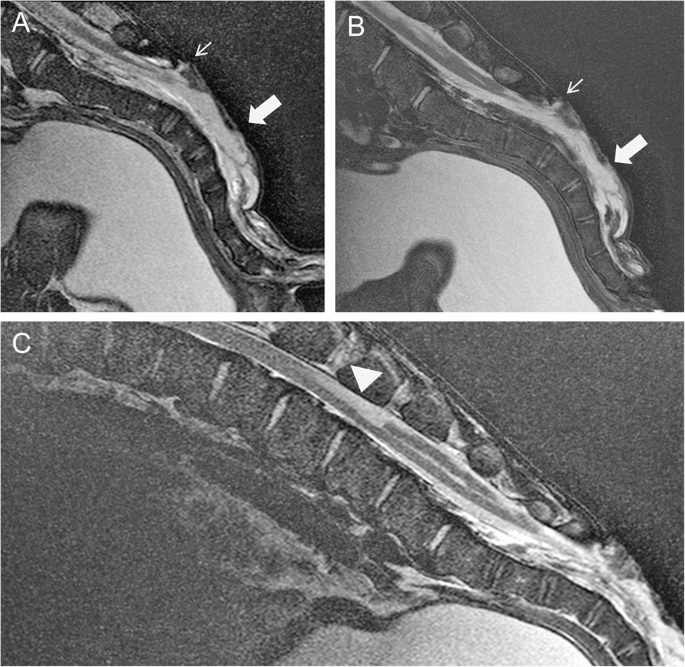
For MRI, general anaesthesia was induced with a combination of 1 ml/100kg xylazine hydrochloride i.m. (Xylavet®, 20 mg/ml, CP-Pharma Handelsgesellschaft mbH, Burgdorf, Germany), 0,3 ml/10kg ketamine hydrochloride i.v. (Ketamin®, 100 mg/ml, CP-Pharma Handelsgesellschaft mbH, Burgdorf, Germany), and 1 ml/100kg butorphanol i.v. (Butorgesic®, 10 mg/ml, CP-Pharma Handelsgesellschaft mbH, Burgdorf, Germany). After administration of ketamine, an intratracheal tube was inserted into the calf’s trachea to maintain anaesthesia with isoflurane (Isofluran®CP, 1 ml/ml, CP-Pharma Handelsgesellschaft mbH, Burgdorf, Germany) in oxygen. MRI with a 3 T scanner (Achieva, Philips Medical Systems, Best, The Netherlands) of the lumbosacral area was performed with the calf in lateral recumbency. Pre- and post-contrast T2 weighted (w) sagittal and transverse sequences, T2w sagittal sequences with spectral attenuated inversion recovery (SPAIR), and T1w sagittal and transverse sequences were obtained. In addition, the head was scanned with the calf in dorsal recumbency using a T2w sequence for sagittal and transverse sequences of the brain up to the 2nd cervical vertebra. MRI of the lumbosacral area revealed the absence of the dorsal lamina of S3 to S5. The spinal canal was filled with hyperintense material, similar to the intensity of cerebrospinal fluid and moderately segmented by membranous structures, in T2w and T2w SPAIR sequences. A hyperintense (T2w, T1w), 2 mm wide connection between the dermis and the epidural space was seen cranial to the lesion at S3. A well-delineated, cranially-anchored, homogeneous, hyperintense lesion was remarkable in the dorsal spinal cord at the level of lumbar vertebra (L) 4 and 5 in the T2w sequence (Fig. 3). The dorsal funiculus caudal to this lesion was partially replaced with cerebrospinal fluid up to the sacral area, where it extended to the sacral segmented lesion. The signal intensity of the lumbar spinal cord was normal. Caudal to the fluid accumulation, the central canal was mildly extended to the lumbosacral transition.
Magnetic resonance imaging of the mid-sagittal lumbosacral area of a 14-day-old Aberdeen Angus calf positioned in lateral recumbency. A and C T2w images. B T2w spectral attenuated inversion recovery. A + B show the absence of the dorsal lamina of the sacrum (S3-5). The vertebral canal is filled with hyperintense material (large arrow). The small arrow points to a hyperintense 2 mm wide connection between the dermis and the epidural space. C At the level of L4/5, a well demarcated T2w in comparison to the surrounding spinal cord tissue homogeneously hyperintense lesion can be detected in the dorsal region of the myelon (arrowhead). The lesion has the extension of a vertebral body and covers up to one third of the spinal cord tissue
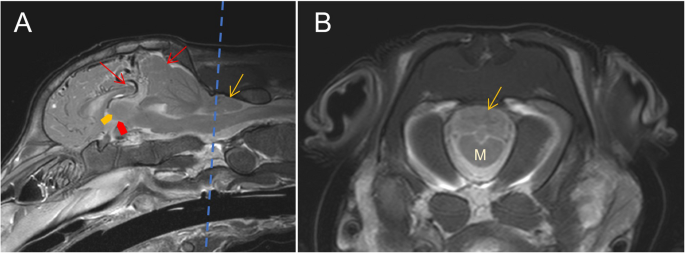
The cerebellar pyramis, uvula, and nodulus were displaced caudally through the foramen magnum 22,63 mm within the spinal canal. The tongue-like structure of the displaced cerebellum penetrated to the cranial quarter of cervical vertebra (C) 1. Caudal subtentorial herniation of the cerebrum was seen. The midsagittal T2w sequence showed a prominent dorsal cerebral sinus and rostral cerebral artery. Diffuse, faintly delineated, hyperintense lesions were seen periventricular to the lateral ventricles and in the brainstem parenchyma in T2w MRI sequences. The latter was at the level of the mesencephalic aqueduct and central canal. The third and fourth ventricles were poorly delineated, appearing as a misshaped interthalamic adhesion (Fig. 4). The cause may have been a secondary ascending inflammation from the S3 connection to the dermis or, more likely, a higher pressure of the cerebrospinal fluid due to the Chiari-like malformation. The intramedullary lesions of the brainstem and ill-delineated interthalamic adhesion are suspected to be due to the cerebellar and subtentorial herniation – as well as the hyperintensity cranial to the cerebellum, most probably caused by a slight dilation of cerebrospinal fluid filled rooms.
Magnetic resonance imaging of the brain of a 14-day-old Aberdeen Angus calf. The dotted line in (A) represents the level of the transverse image in (B). A T2w mid-sagittal image shows severe herniation of the cerebellum through the foramen magnum (yellow arrow) and subtentorial herniation of the cerebrum (red arrows). The third ventricle (red bold arrows) and the adhesio interthalamica (yellow bold arrow) are misshaped. B T2w transverse image at the level of the foramen magnum shows the medulla oblongata (M) with parts of the cerebellum (yellow arrow)
Euthanasia
The owner consented to euthanasia of the calf because of a grave prognosis. Euthanasia was performed immediately after MRI at day 14, with the calf still under general anaesthesia, by intravenous administration of 3 ml/10kg pentobarbital-sodium (Release®, 300 mg/ml, WDT, Garbsen, Germany). Euthanasia was conducted according to the method recommended by the Ethics Committee of the Lower Saxony State Office, Veterinary Institute Hannover.
Postmortem findings
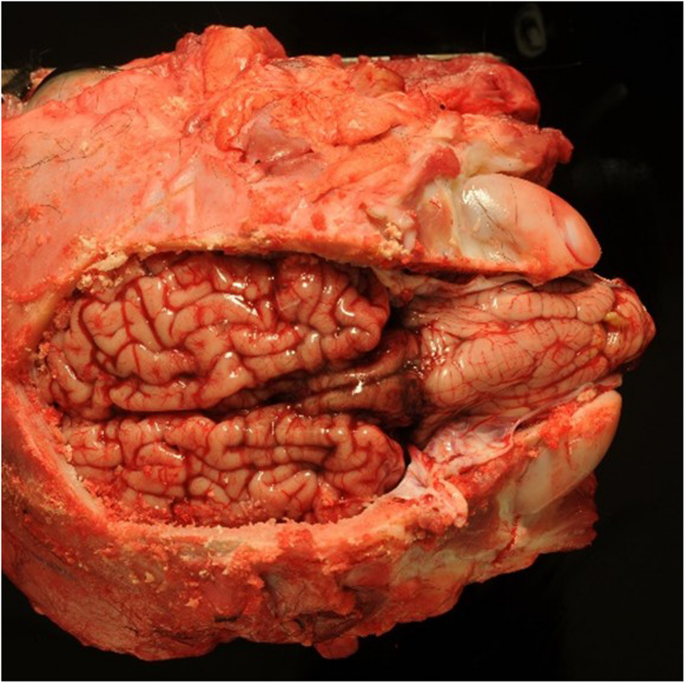
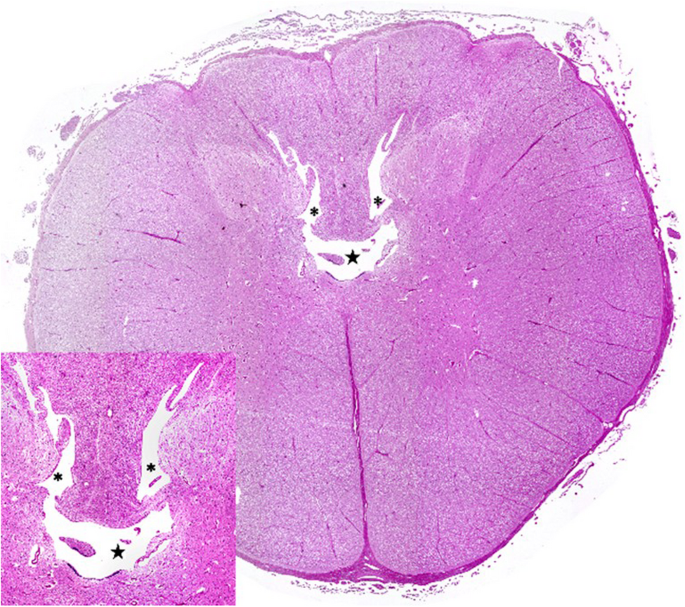
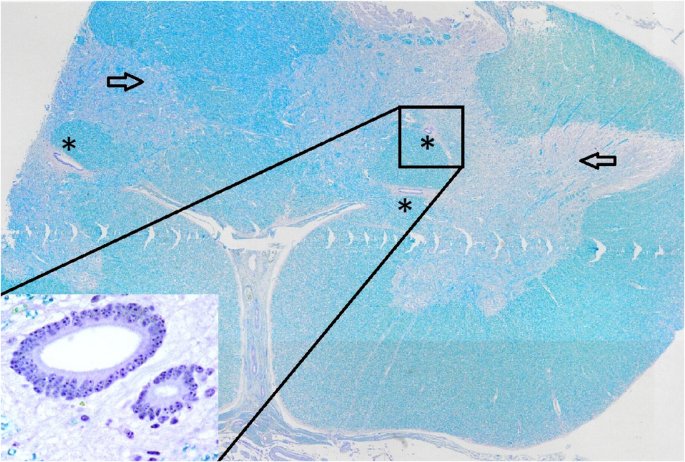
Postmortem examination showed severe herniation of the cerebellar vermis into the foramen magnum with associated marked congestion (Fig. 5). The vertebral arches of S3, S4, and S5 were incompletely developed. A 6-cm, circular, hairless area was present at the S3 to S5 midline. Tissue samples were fixed in 10% neutral buffered formalin, embedded in paraffin wax, sectioned, and stained with haematoxylin and eosin (H&E) and luxol fast blue stains. Histological examination of the cervical spinal cord revealed dilatation of the central canal (hydromyelia) and bilateral cavitation of the spinal cord (syringomyelia) (Fig. 6). Transverse sections of the sacral spinal cord showed duplication of the grey matter and central canals (diplomyelia). Additional central canals with a smaller diameter were seen, the smallest measuring 50 μm. Enlargement of the ventral median fissure was present at this location (Fig. 7). The alopecia at the sacral midline area (S3–S5) was characterised by diffuse epidermal ulceration with mild neutrophilic infiltration and granulation tissue formation. In the underlying tissue, mild infiltration of neutrophils, macrophages, and lymphocytes was observed in the arachnoid membrane. Moderate epidermal hyperplasia and mild orthokeratotic hyperkeratosis were seen adjacent to the area of alopecia. Severe suppurative myositis was seen ventral to the area of alopecia. A RT-PCR assay for SBV RNA of spleen and brain tissue carried out by the Lower Saxony State Office, Veterinary Institute Hannover, was negative.
Dorsal view of the brain showing severe herniation, elongation and compression of the cerebellar vermis into the foramen magnum
Transverse section of the cervical spinal cord with severe dilatation of the central canal (hydromyelia) and bilateral cavitation of the spinal cord (syringomyelia). The insert shows hydromyelia (★) and syringomyelia (*) in detail. Haematoxylin and eosin stain
Transverse section of the sacral spinal cord showing duplication of the grey matter (arrow) and multiple central canals (*). The insert is a higher magnification of the central canals. Luxol fast blue stain
Cytogenetic analysis
Cytogenetic analyses were performed to identify chromosomal aberrations. The chromosome preparation followed standard protocols using heparinised blood samples for lymphocyte culture preparation [20]. The metaphase chromosomes were stained with Giemsa and examined with a light microscope (Axioplan 2, Carl Zeiss Microscopy, Jena, Germany). Photos were taken with a computer-controlled CCD camera (Axiocam 105 color, Carl Zeiss Microscopy) and processed with ZEN 2 (blue edition) software (Carl Zeiss Microscopy). A total of 50 metaphase chromosomes were analysed, all displaying a normal karyotype with 2n = 60, XX chromosomes. Numerical or chromosomal aberrations of autosomal and sex chromosomes were not observed in any of the metaphase chromosomes.
Whole genome sequencing
For whole genome sequencing (WGS), genomic DNA was isolated from an EDTA-blood sample of the affected calf and semen samples of the sire of the affected calf using standard chloroform extraction. According to the manufacturer’s protocols libraries from these samples were prepared using randomly fragmentation of 1µg genomic DNA. Thereafter, selection by Magnetic beads to an average size of 200–400 bp were done. Fragments were end repaired and then 3’ adenylated. Adaptors were ligated to the ends of these 3’ adenylated fragments. PCR followed to amplify fragments with adaptors from previous step. PCR products were purified by the Magnetic beads. Denaturation and circulation following by the splint oligo sequence. Size selection and indexing was quality controlled on an Agilent 2100 Bioanalyzer system with a High Sensitivity DNA kit (Agilent Technologies, Santa Clara, CA). WGS was performed using a DNBSEQ-T7 (formerly known as MGISEQ-T7) in a 2 × 150 bp paired-end mode. Quality control for the data was performed using fastqc 0.11.9 [21] and the reads were trimmed using SOAPnuke software (-n 0.001 -l 10 -q 0.4 -A0.25 -Q 2 -G –cutAdaptor –minLen 150) (BGI, HongKong, China). The resulting data were mapped to the bovine reference genome ARS-UCD1.2 (Ensembl) using BWA 0.7.17 [22] resulting in a mean coverage of 19.5X for the case (average mapping rate 99.5%) and 19.7X for its sire (average mapping rate 99.4%) and 10-19X for the controls. SAMtools 1.11 [23] and Picard tools (http://broadinstitute.github.io/picard/, version 2.25.0) were used for sorting, indexing and marking of duplicates of bam-files. Variants were called with GATK, version 4.2.0.0 [24], using Base Quality Score Recalibration (BQSR), Haplotype Caller and Variant Recalibrator. The variants of the two sequenced animals were compared with variant callings from 34 private controls including cattle of the breeds Holstein, Fleckvieh, Braunvieh, Vorderwald, German Angus, Galloway, Limousin, Charolais, Hereford, Tyrolean Grey and Miniature Zebu. All variants with high or moderate effects according to prediction toolbox SNPEff, version 5.0 e (2017-08−30, SNPEff database ARS-UCD1.2_release 103) [25] were selected. The WGS data of the affected calf and its sire were screened for mutant variants using SAS, version 9.4 (Statistical Analysis System, Cary, NC, USA). We filtered out variants homozygous for the affected calf and heterozygous for the sire for the case of a recessive inheritance, or heterozygous for the affected calf but not shared by the sire for the case of a spontaneous mutation or germline mutation. Predicted effects of these private variants were investigated using the Variant Effect Predictor [26] for SIFT [27] predictions and PolyPhen-2 [28]. All variants were searched for candidate genes based on the literature. A keyword search for publications related to spina bifida in humans or domestic animals and based on the clinical signs observed in the affected calf with searching of the following words ‘cerebellar displacement’, ‘arnold-chiari malformation’, ‘type II chiari malformation’, ‘arnold-chiari-like’, ‘dysraphic disorder’, ‘spinal dysraphism’, ‘occipital encephalocels’, ‘chiari-like malformation’, ‘caudal occipital malformation’, ‘occipital hypoplasia’, ‘hydrocephalus’, ‘internal hydrocephalus’ or ‘meningomyelocele’ to detect genes associated with such phenotypes was conducted using NCBI gene (https://www.ncbi.nlm.nih.gov/gene) and Ensemble (https://www.ensembl.org/index.html). The purpose was to find variants in the calf but absent in the sire or variants in the calf and the sire but absent in 34 control samples from 11 different breeds. Reads were mapped to the ARS-UCD1.2 bovine reference genome, resulting in a mean coverage of 19.5X for the calf, 19.7X for the sire, and 10-19X for the 34 controls. The calf, sire, and 34 control samples had 34.288.637 single base pair variants and 5.513.879 indels. The calf had 40.138 variants affecting protein function (additional file 1), and the calf and sire had 27.496 (additional file 2). Homozygous mutated variants in the calf and heterozygous variants in the sire yielded 1588 results (additional file 3). A total of 46 variants remained after filtering for variants with high or moderate effects and variants associated with genes related to the following diseases (additional file 4): cerebellar displacement, Arnold-Chiari malformation, type II Chiari malformation, Arnold-Chiari-like malformation, dysraphic disorder, spinal dysraphism, occipital encephalocele, Chiari-like malformation, caudal occipital malformation, occipital hypoplasia, hydrocephalus, internal hydrocephalus, and meningomyelocele (additional file 5). This procedure was done according to SNPEff predictions on protein structure. The intronic splice acceptor variant (g.19087428 C >CTTGATAT, c.879-1_879insATATCAA) within GRM7 on BTA22 (ARS-UCD1.2) was the most likely candidate due to its protein impact through altered splicing.