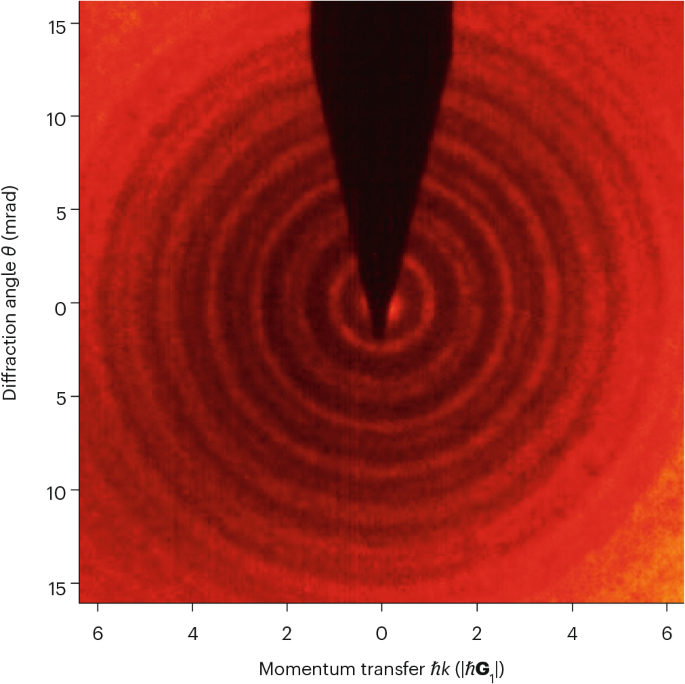
Diffraction is a wave phenomenon that occurs when a wavefront encounters matter or a slit with feature dimensions comparable to its wavelength. The wave spreads out and interferes with itself, generating a pattern of high and low wave intensity. Owing to wave–particle duality, the particles can interact with a periodic structure with characteristic length comparable to their de Broglie wavelength, resulting in a pattern of high and low probability for particle detection.
This phenomenon of quantum particle diffraction is the foundation for many analytical techniques to probe the structure of matter at the atomic scale, such as X-ray diffraction, transmission electron microscopy, low-energy or reflection high-energy electron diffraction, neutron diffraction, and helium atom scattering. For a beam to transmit and diffract through a solid, its interactions with the solid lattice must be sufficiently weak to suppress absorption or pronounced scattering — conditions that are inherently difficult to achieve for atoms, given their relatively larger size and stronger interatomic interactions compared with subatomic particles such as electrons.