General aim
The project aims to identify frail older individuals early, through a multiple steps process (first level screening and multidimensional assessment – second level screening) applied to 75-year-old adults in a specific Italian neighborhood. The specific process represents an adaptation of the ICOPE model and will enhance the definition of a tailored, multicomponent pathway for each patient, based on individual needs and the collaboration among various PC professionals and general practitioners (GP).
Specific aims
To determine the prevalence of frailty among 75-year-old adults using a first level screening tool, providing baseline data to define the subsequent pathway of care.
To analyse the characteristics and health outcomes of frail individuals, and to identify the determinants associated with these outcomes, within the individuals who will test positive to first level screening and will undergo the multidimensional assessment (second level screening), including determinants of health status influencing frailty progression.
To explore the relationship between HL and frailty, examining how HL influences with outcomes from multidimensional assessment.
Study design and setting
The study is a single-center prospective longitudinal cohort study. It will take place in a House of Community (HoC – “Casa della Comunità”) located in the most populous neighborhood of the city of Florence, in the Tuscany region. This facility represents a specific model of Community Health Center, focused on enhancing continuity of care and integration among health professionals and services through multiprofessional collaborative practice, and promoting HL, health promotion activities and community engagement [21]. The study includes two specific phases: a first level screening and a following second level multidimensional assessment.
This protocol was drafted adhering to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) [22].
Study participants and eligibility criteria
The study population is represented by all 75-year-old persons living in a specific area of the Florence Health District – the reference area of the chosen HoC Le Piagge, where the screening and the other interventions will be realized – who voluntarily decide to participate and are apparently self-sufficient and not physically or mentally disabled. The choice of this age group is due to epidemiological reasons – that is a higher prevalence of frailty in 75–84-year-old compared to the younger [9, 23, 24], together with the fact that the screening must not identify an excessive number of negative individuals (healthy, without signs of frailty) and, at the same time, must be able (by means of a multidimensional assessment) to identify conditions of initial frailty, so as to be able to intervene (also) with early and non-pharmaceutical options. Other reasons are the organization of preventive strategies in Italy that are often targeted to this age group and the feasibility reason linked to the study design.
The design will provide for the annual recruitment of 75-year-old people to follow them and the cohorts recruited in previous years, so that the monitoring of people can reveal potential signs of loss of autonomy over time.
In the first year of the study, more than 400 subjects (after extracting all the 75-year-old people living in the area of the HoC and its surroundings from the municipal registry) will be contacted for participating in the study. A priori sample size was not estimated because participation was offered to all resident 75-year-old individuals. This is a pilot study, and its primary aim is to assess the applicability and acceptability of the model proposed. However, in order to ensure transparency, an estimate of expected recruitment has been added on the approximate number of residents in the area and the expected participation rate (of almost 20–30%). Moreover, an attrition rate of approximately 20% will also be considered, mainly due to loss of contact, refusal, or health-related reasons.
Participants are eligible if they are 75-year-old at the time of recruitment, if they accept to sign a written informed consent and are able to read and understand spoken and written Italian language, as the assessment tools are all in Italian.
The following conditions represent exclusion criteria, that will be verified during the recruitment phone call, through direct questioning of the potential participants or their caregivers, and also at the first appointment for the screening: being resident in long-term care or being non-self-sufficient, and the presence of severe cognitive impairment, severe psychiatric disorder, terminal illness, or any other condition of disability, since all these conditions are not compatible with early detection of frailty.
This study complies with the Declaration of Helsinki.
Data collection process
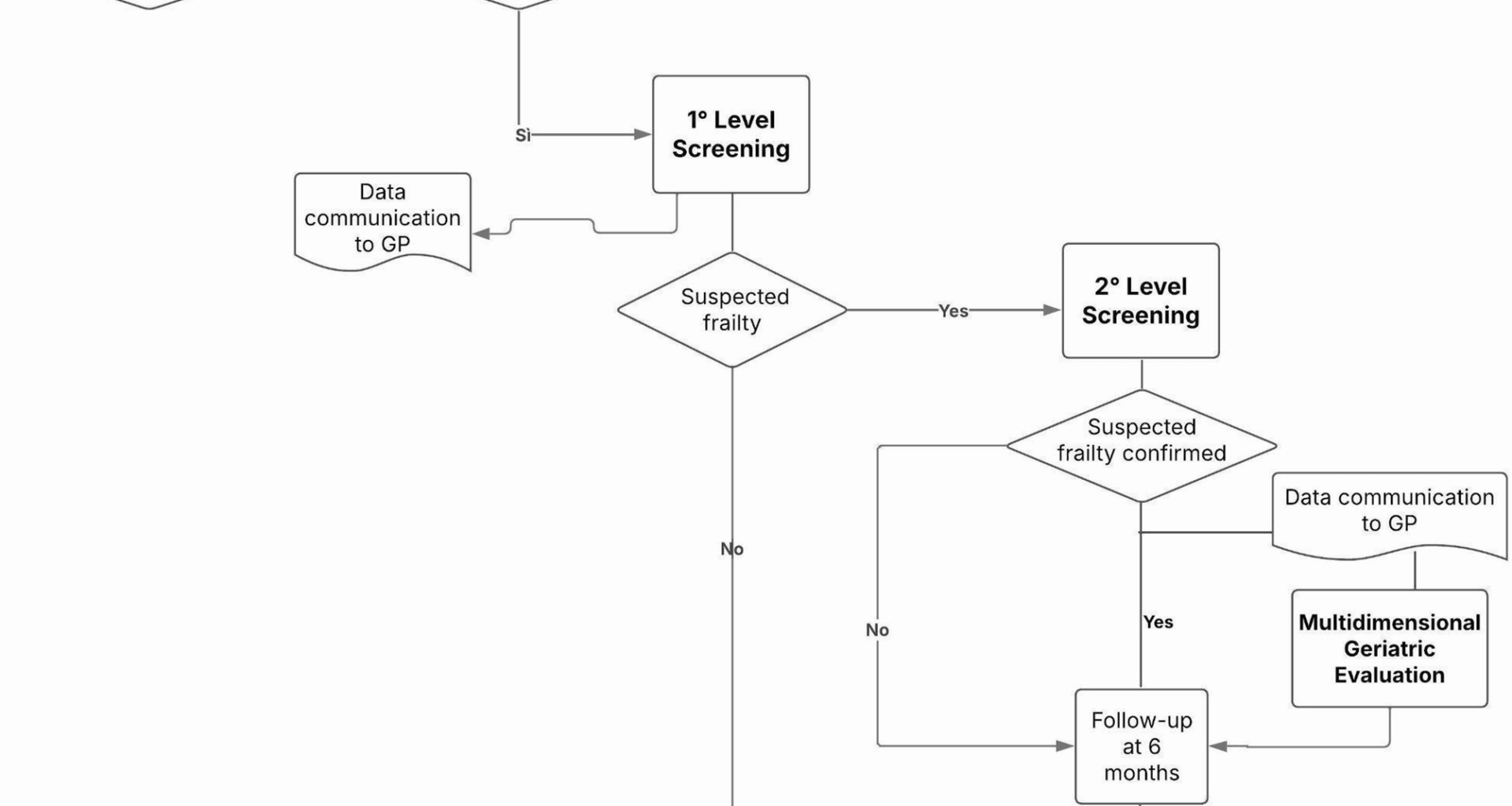
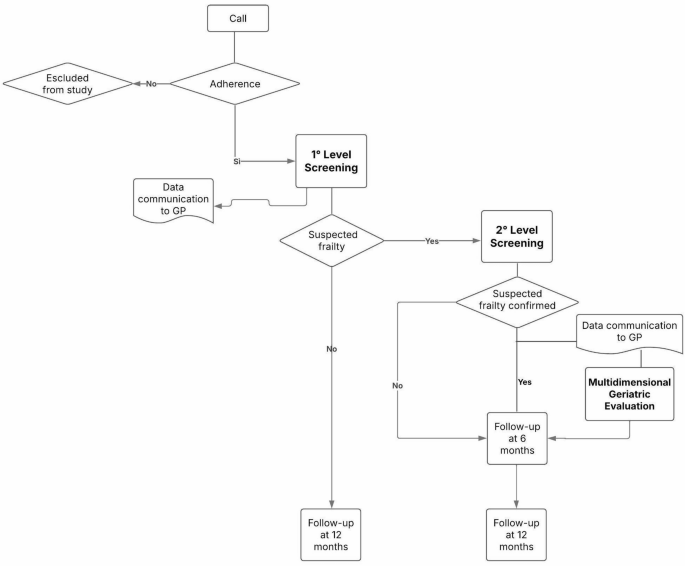
After extraction from the municipal register, the subjects who meet the inclusion criteria will be contacted by phone and invited to participate by a group of researchers, specifically trained for all the data collection phases, and after informing the GPs operating in the area. For the telephone contacts, the numbers were extracted from the Local Health registry. The telephone contact, repeated up to 3 times and providing a specific phone number for call-backs, will serve as an initial explanation of the study, to acquire a verbal consent for participation and to improve its rate. This phase – the recruitment – is planned to last approximately two months. Those who accept to be included are offered an appointment at the HoC where they can complete the first-level screening (2 months) with the support of the multidisciplinary research team, after signing the informed consent for participation and data collection and processing. In addition, a digital support has been specifically developed to make the self-administration of the test easier. The results of the first level screening will allow participants to be classified into two groups: those who test negative are called back for follow-up screening in the following year, accounting for the fact that after the age of 75 any person may experience a loss of autonomy within 12 months, while those who test positive are likely to be “frail” and are invited to a subsequent appointment for the second, more in-depth level test – a multidimensional assessment focused on various frailty domains, administered by the specifically trained research team (4 months). The results of the second screening will highlight in which domains the subject is most at risk of frailty. Based on the results of this multidimensional assessment, and in presence of at least one positive domain, the GP – who will be informed step by step of all the results of his/her patients – may decide for a specialist geriatrician examination, which is offered free of charge at HoC ‘Le Piagge’. The geriatrician further evaluates the patient’s health needs and care complexity and collaborates in drawing up a personalized care plan, together with other PC professionals and the GP himself. Follow-ups for the multidimensional assessment are scheduled at 6 and 12 months after the initial screening (Fig. 1). The successful conclusion of the follow up at 12 months marks the end of the study. Participants drop-out will be monitored and documented at each phase (first-level screening and multidimensional assessment). Reasons for drop-out will be collected (refusals, health conditions, loss to follow-up). Different strategies will be implemented to minimize drop-out, such as the provision of reminders to participants, the offer of flexible scheduling, alternative times for assessment and the involvement of GPs to encourage participation.
Depending on participation rates and preliminary outcomes, the feasibility of extending recruitment to subsequent cohorts of the same age group will be evaluated. The study is expected to conclude within two years from the start of recruitment, including also the data analysis and the reporting and dissemination of results.
Outcomes’ measure
Prevalence of frailty in terms of individuals identified as potentially frail based on the first-level screening.
Distribution of frailty domains in terms of identification of specific frailty domains affected (e.g. cognitive, functional, nutritional, psychological, social) as measured by the multidimensional assessment tools.
Baseline HL scores and their relationship with frailty status.
Analysis of baseline characteristics that may predict frailty status or its progression.
Identification of common co-occurrences of frailty domains (e.g., cognitive and nutritional domains).
Variables and tests used
Data collected during the study will include the following variables and tests. The timing of all assessments is summarized in Table 1. Regarding demographic variables, the following data will be collected: date of birth, sex, citizenship, first language, place of residence, educational level, employment status, economic condition, marital status, living and family arrangement.
Table 1 Timing of the different tests at both study levels
First level screening includes the following specific tests:
Frailty screening test to detect the presence of frailty: a validated questionnaire developed by the Tuscany regional commission [19, 20], which evaluates the vision (e.g., ability to read newspaper headlines with or without glasses); fatigue during daily activities; memory problems; history of falls within the last six months; difficulty walking 400 m on flat terrain; regular intake of five or more drugs. This test is a proactive screening model, scoring from 0 to 11.5 and validated in a Tuscan population, with higher score indicating higher likelihood to be frail, and a frailty cut-off fixed to 6.5.
The Chair Rise Test, as suggested in the ICOPE framework, will be added to assess lower limb muscle strength [16].
Single Item Literacy Screening (SILS), a subjective measure of HL, which is based on highlighting difficulties in reading medical material. It asks: “How often do you need help when reading instructions, pamphlets, or other written material from your doctor or pharmacist?” [25].
Second level screening: multidimensional assessment.
Mini Cog Test, which is a screening tool comprising two components – a short-term memory task and the clock drawing test for visual-spatial and executive function. It evaluates both short- and long-term memory, visual-spatial representation, attention, and executive functions and it is developed as a brief test to identify dementia in culturally, linguistically, and educationally diverse older adults [26, 27].
Short Physical Performance Battery (SPPB), which aims to evaluate physical performance by assessing the balance, the gait (in terms of speed over a short distance) and the muscle strength and endurance [28,29,30,31].
Basic Activity of Daily Living (BADL), assessing six parameters of daily life (bathing, dressing, toileting, transferring – e.g., bed to chair, continence – bowel and bladder, eating), providing an objective measure of a patient’s autonomy level [32].
Instrumental Activity of Daily Living (IADL), assessing the ability to perform complex activities that are normally carried out also by older adults required to maintain their full independence [33].
Malnutrition Universal Screening Tool (MUST), that is a screening test for evaluating the nutritional risk, developed by the BAPEN (British Association for Parenteral and Enteral Nutrition) Malnutrition Advisory Group, reviewed regularly since its launch in 2003 [34, 35].
Strength, Assistance in walking, Rise from a chair, Climb stairs, and Falls (SARC-F5), which represents the screening test for sarcopenia, involves the following dimensions: strength, support in walking, getting up from a chair, climbing stairs and falls [36].
WHO simple eye chart, to evaluate vision loss [5].
Hearing Handicap Inventory for the Elderly (HHIE-It), which explores the emotional consequences of hearing loss and its social and situational effects [37].
Geriatric Depression Scale (GDS-5): it includes an evaluation of affective, cognitive and behavioural symptoms related to depression in older adults, as defined by DSM IV and ICD-10 [38, 39].
European Health Literacy Survey Questionnaire 16 items (HLS-EU-Q16) a subjective tool that measures three components of HL: functional, critical, interactive. It permits the evaluation of accessing, understanding, appraising and applying information on healthcare, health prevention and health promotion [40].
Newest Vital Sign (NVS), an objective tool which plans to measure the functional HL [41].
Statistical analysis
Descriptive statistics will be used to summarize the sample’s general characteristics, including health-related variables. This initial analysis will help identify potential confounders. Subsequently, bi-variate analysis (t test and chi-square test) will be conducted to compare the differences between frail and non-frail individuals, based on tool scores. Additional comparisons will be made between individuals classified as frail based on the multidimensional assessment. Regression models will be applied to the subgroup with positive first-level screening (who undergo multidimensional assessment) to examine associations between demographic variables and results from the multidimensional assessment. Potential confounding variables will be accounted for in the models, and outcomes will be adjusted accordingly. A comparative analysis of HL tools will be used to evaluate their capacity to identify HL levels and to predict frailty. Roc curve analyses will be conducted to compare the predictive ability of each tool. Considering the longitudinal design of the study, data will be analysed at specific time points corresponding to baseline and subsequent follow-up assessments.
In analysing changes over time for each secondary frailty domain, it will be considered the established thresholds for minimal clinically important differences (MCIDs) for tools where it is defined. For other outcomes (e.g., Mini-Cog, MUST, IADL), descriptive and categorical analyses will be used. When changes will be observed across clinically defined thresholds (e.g., from “low” to “high” nutritional risk), these will be reported and interpreted accordingly. Missing data will be examined performing a descripting analysis of the type and patterns and using a Multiple Imputation by Chained Equations for missing covariates and outcomes. 0Ethical considerations and dissemination.
The protocol has been approved by the Ethics Committee of the Tuscany Region – sezione Area Vasta Centro, on 05/28/2024, version 1st. Any important protocol modifications will be timely communicated to relevant parties, such as the Ethics Committee. A specific Data Monitoring Committee was not established for the study because of its observational design and the absence of interventions planned with low risks for participants. However, principal investigators and the local Ethics Committee will monitor the implementation. The research team will provide all participants with a complete explanation of the study and obtain written informed consent before enrollment. Participants have the right to withdraw from the study at any time and will be directly involved to ensure their well-being and to prevent any source of stress. This study is in compliance with the Declaration of Helsinki. All collected data will be stored in a password-protected database, accessible only to authorised research team members. Personally identifiable information will be replaced with unique participant codes to ensure confidentiality. Data will be processed and analysed in accordance with applicable data protection regulations, including the General Data Protection Regulation (GDPR). The complete documents – protocol and informed consent – will be available under request. Manuscript does not contain any data. The findings will be presented at national conferences and in peer-reviewed journals.