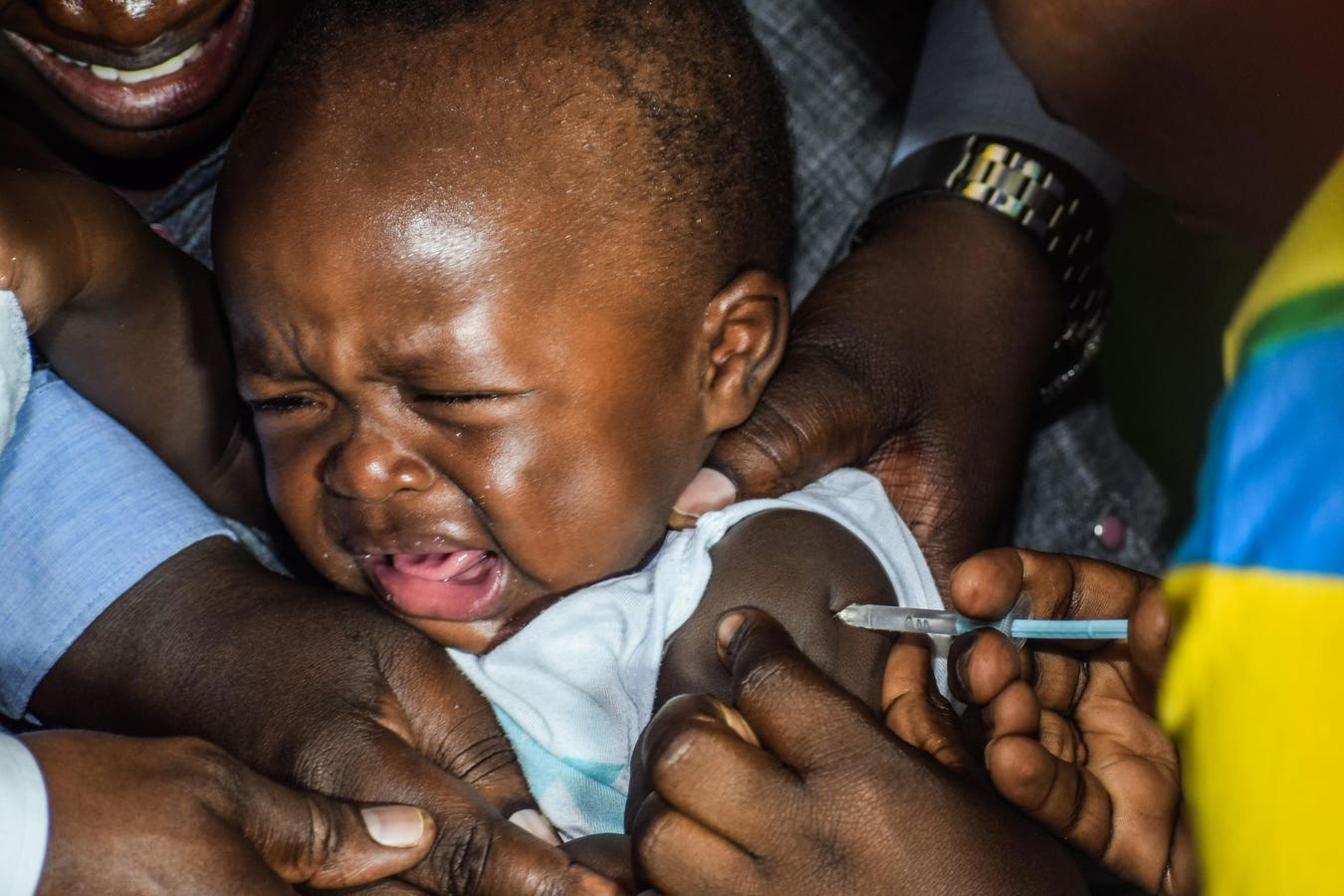
A child is vaccinated against malaria on September 13, 2019 in Ndhiwa, Homabaya County, Western Kenya during the launch of a malaria vaccine in Kenya. The vaccine (Mosquirix) is the world’s first malaria vaccine that has been shown to provide partial protection against malaria in young children and has been rolled out by World Health Organization in Kenya, Ghana and Malawi. (Photo by Brian ONGORO / AFP) (Photo by BRIAN ONGORO/AFP via Getty Images)
AFP via Getty Images
A late stage trial for an investigational malaria drug called GanLum (ganaplacide/lumefantrine) has demonstrated a 97.4% cure rate, outperforming currently existing treatments. The drug is being developed by the pharmaceutical firm Novartis in collaboration with a not-for-profit foundation called the Medicines for Malaria Venture. If approved, GanLum will be considered an important advance against treatment-resistant malaria strains.
Malaria is a mosquito-borne disease which carries with it a particularly high disease burden in children in Africa. In the 20th century alone, malaria claimed between 150 and 300 million lives globally. Annually, nearly 600,000 people die from the communicable disease worldwide, more than 500,000 of whom are in Africa. The vast majority of victims are younger than five years old.
Since around 2000, one of the major success stories in global public health has been the emergence of improved anti-malarial treatments and vaccines. Coupled with preventive efforts boosting the distribution and use of mosquito nets, pharmaceuticals have helped lower the annual death toll from malaria by around 30%, from around 850,000 to 595,000.
GanLum’s Phase 3 data presented at this year’s American Society of Tropical Medicine and Hygiene annual meeting highlight the drug’s potential to further combat the threat of antimalarial drug resistance in Africa. Besides GanLum, Novartis has other next-generation malaria medicines in its pipeline that target the increased resistance to existing treatments.
Quinine was first used to treat malaria nearly 400 years ago. But it took until the 1930s for a better therapeutic to be developed, chloroquine. Forty years later, Chinese scientists, including the country’s first Nobel Prize winner in medicine, Tu YouYou, discovered that artemisinin could be used to target a main causal agent of malaria. Artemisinin became the base for a series of anti-malarial therapies. By the 1980s, artemether, for example, was the main line of defense against treatment-resistant malaria.
The next step in the evolution of antimalarial drug development was the emergence of fixed-dose combination products, such as Coartem, first used in 1999. It is a combination of the antivirals artemether and lumefantrine. The World Health Organization recommends artemisinin or one of its derivatives―typically in combination with another drug―as frontline therapy for malaria. Coartem is one of five WHO-recommended artemisinin-based combination therapies.
Coartem has undergone several iterations since 1999 in terms of formulation, designed to facilitate the treatment’s uptake in children. The very latest version is called Coartem Baby. It recently became the world’s first approval for a medicine to treat babies who are infected with malaria. Coartem Baby is dissolvable, can be taken with breast milk and has a sweet cherry flavor. The drug’s sponsor, Novartis, plans to introduce the treatment on a largely not-for-profit basis to increase access in areas where malaria is endemic.
It was around the time that Coartem first launched that the WHO profiled the “big three” infectious diseases, HIV, tuberculosis and malaria, as the most deadly in the developing world. This led to the creation of the Global Fund to Fight AIDS, Tuberculosis, and Malaria which mobilized substantial resources to advance prevention and treatment of all three diseases. The work of the Global Fund along with a commitment of research and development funds for malaria have borne fruit in the past two decades, in the form of treatments but also vaccines.
Mosquirix, RTS, S/AS01, is a recombinant protein-based vaccine. The vaccine was originally developed in 1987, but it took more than 30 years for a pilot program to commence in endemic countries in 2019. In the areas where Mosquirix was tested, infant mortality fell by 13%.
The WHO awarded prequalification status to Mosquirix in 2021, which meant that United Nations agencies such as UNICEF could purchase the vaccine in partnership with Gavi, the Vaccine Alliance, and eligible countries. Clinical researchers and healthcare workers expect it to save tens of thousands of children’s lives. The WHO has also recommended a second malaria vaccine, R21/Matrix-M, which is less expensive.
In 2026, demand across Africa may reach 40 to 60 million doses of the two vaccines. The roll-out of the malaria vaccines is now well underway. By early April 2025, 19 countries had introduced the vaccine as part of routine childhood vaccinations, with scale-up planned throughout this year.
Further expansion of uptake could be augmented by an agreement signed last week by UNICEF (the world’s largest buyer of vaccines) and Gavi (an alliance that assists lower-income countries in their purchase of vaccines targeting a variety of diseases). The groups say this will help procure affordable malaria shots to be administered to seven million more children over the next five years.
It’s not all good news. A WHO report warns that progress in combating new cases of malaria is in peril, particularly in light of the Trump administration’s funding cuts affecting the President’s Malaria Initiative, an organization established by former President George W. Bush. Through the work of this entity, the United States had become the world’s largest donor to anti-malaria programs and research. According to the New York Times, one of Trump’s executive orders has led to two-thirds of the staff being let go from the Malaria Initiative.
But for now, it’s worth celebrating achievements such as GanLum in the fight against the scourge that is malaria. A doctor on Twitter noted that just “in the last year there have been breakthroughs for vaccines, transmission-blocking innovations, better surveillance and new and improved drugs. Step by step, we’re slowly moving towards malaria eradication.”