The U.S. Food and Drug Administration is warning consumers about a nationwide recall involving nasal sprays that could lead to life-threatening illness.
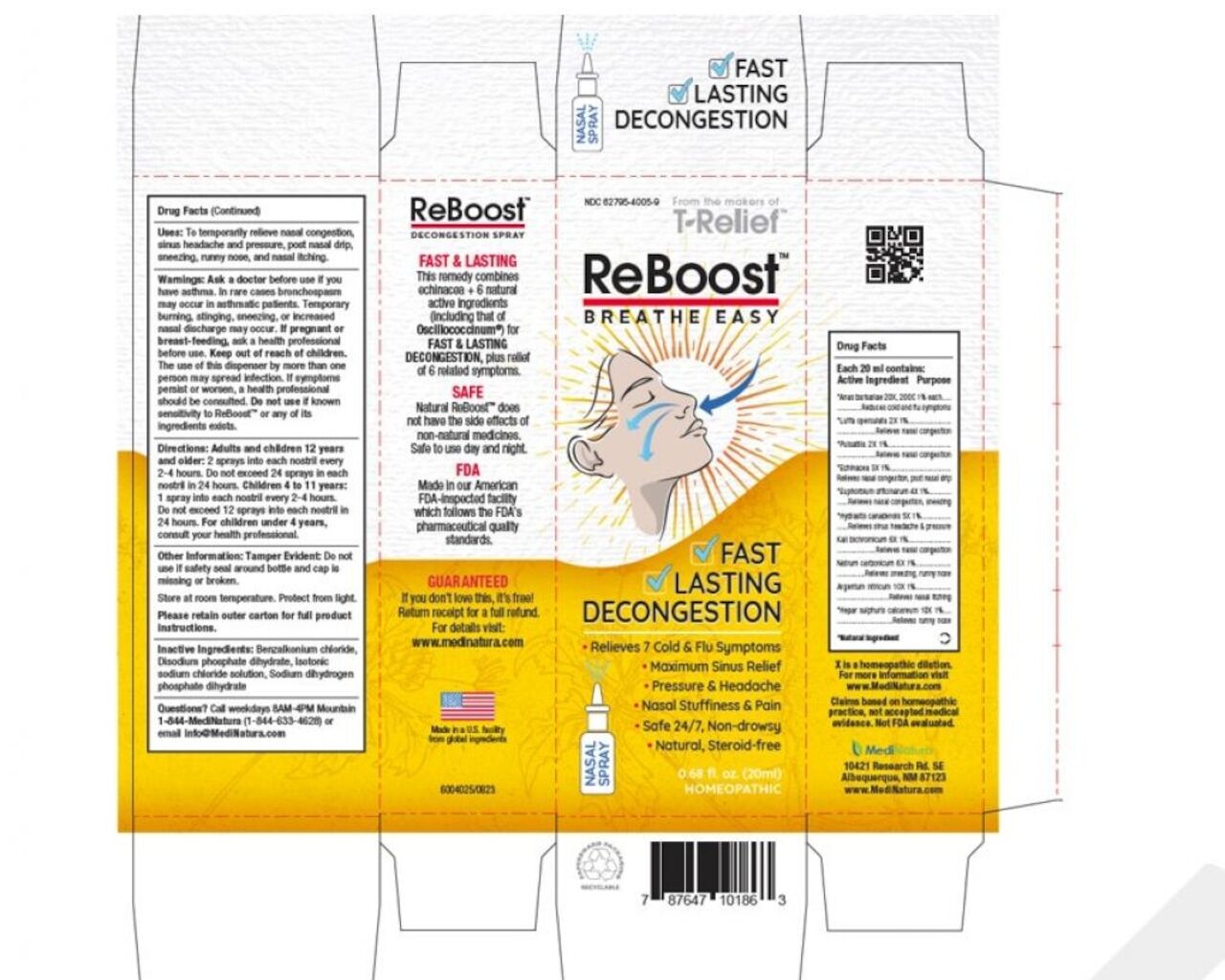
MediNatura New Mexico Inc. is recalling one lot of ReBoost Nasal Spray. The product has been found to contain yeast/mold and microbial contamination, with one species identified as Achromobacter, at levels above specifications.
This could lead to adverse health consequences, including life-threatening infections that could occur with use of the product in the immunocompromised population.
To date, MediNatura has not received any reports of adverse events related to this recall, according to the notice.
The product is used as a homeopathic nasal spray to temporarily relieve nasal congestion, sinus headache and pressure, post-nasal drip, sneezing, runny nose, and nasal itching.
It is packaged in a 20mL bottle which is further packaged in a white and yellow carton. The recalled products have UPC number is 787647 10186 3, and lot number 224268. They have an expiration date of December 2027.
The product was distributed nationwide via retail and internet sales at medinatura.com.
All consumers are urged to immediately discontinue using the recalled ReBoost. Customers who purchased directly from the company should contact MediNatura at recall@medinatura.com for a refund. Consumers who purchased the item at retailers should return it to the place of purchase.
Consumers with questions regarding this recall can contact MediNatura New Mexico Inc. at 800-621-7644 or email recall@medinatura.com.
Consumers should contact their health-care provider if they have any issues that may be related to taking or using this product.