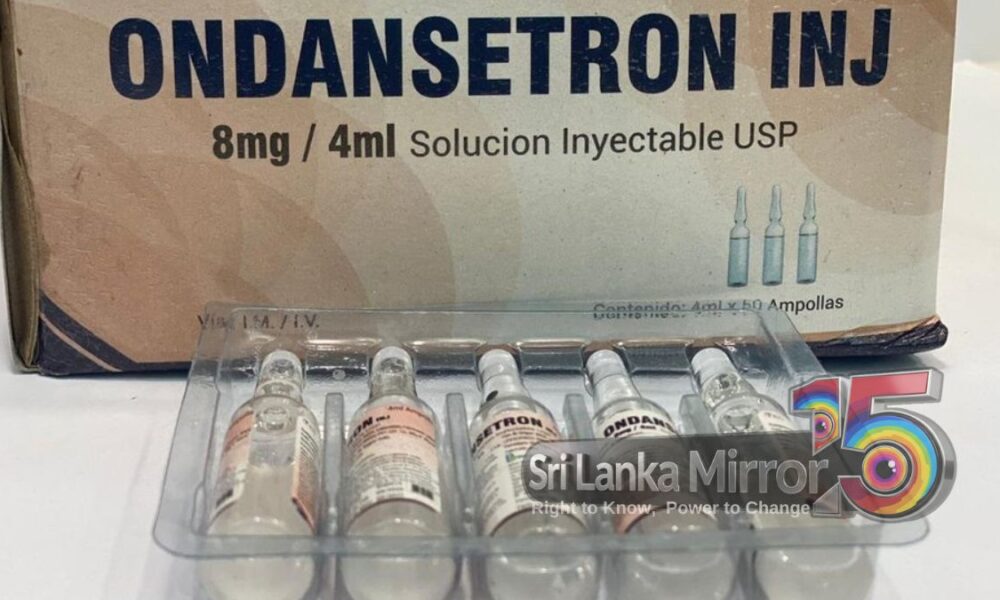
The National Medicines Regulatory Authority (NMRA) has temporarily withdrawn several batches of the Ondansetron Injection (Ondanman 8, USP 8 mg/4 ml) with immediate effect.
The move follows a recently reported incident at the Infectious Diseases Hospital (IDH), where a patient developed complications after receiving the injection, which had subsequently led to the patient’s death.
NMRA Chairperson – Dr. Ananda Wijewickrema clarified that while the death occurred after administration of the injection, it cannot be conclusively attributed to the medicine.
The Ondansetron injection was imported by PTC Holdings (Private) Limited and its subsidiary, PTC Medical (Private) Limited.
PTC Medical (Private) Limited has been registered under NMRA number M012380 for a 05-year period since October 2022.
The product was reportedly sourced from MAAN Pharmaceuticals Ltd., India.
Ondansetron Injection is commonly used to prevent nausea and vomiting associated with surgery, anesthesia, cancer chemotherapy, radiation therapy, and, in some cases, among pregnant women and children, according to medical professionals.
While social media reports claimed 02 patient deaths linked to Ondansetron injections at the Kandy National Hospital, Acting Director of the hospital – Dr. Chandana Jayantha clarified that no reports had confirmed that the deaths were directly caused by the injection.
According to an official letter (NMRA/PA/I/ADR/04/2025) dated Dec. 12, 2025, issued by the Executive Director of the NMRA – Dr. K.M.G.K. Bandara, the withdrawal follows reports of complications and adverse reactions linked to the use of the injection.
The letter is shown below.

Meanwhile, medical associations have raised concerns that imported drugs, approved under Indian Pharmacopoeia (IP) standards, are not being retested locally.
President of the Medical and Civil Rights Professional Association of Doctors (MCPA) – Dr. Chamal Sanjeewa, stated that after the current government assumed office, regulatory provisions were introduced allowing medicines approved by the Indian Pharmacopoeia (IP) to be used in Sri Lanka’s hospital system without additional local testing, and urged authorities to establish procedures to retest such imported medicines in Sri Lanka before use.
He stated this at a media briefing held in Colombo yesterday (Dec. 14).
On their website, PTC Holdings (Private) Limited has named Mr. Gihan Beneragama, Mrs. Gimhana Wickramasurendra, and Mr. Jaliya Wickramasurendra as their Director Board members.
Mr. Gihan is the son of former NMRA Director Dr. Hemantha Benaragma.
This marks the second regulatory concern involving the company, following issues with a cataract lens tender 02 years ago.
📌Related News: