More than 670 medicines are currently affected by supply disruptions in Finland, with many pharmacies unable to provide common over-the-counter and prescription treatments. The Finnish Medicines Agency (Fimea) updated its shortage register on Friday morning, showing widespread gaps in availability.
The list includes familiar pain and fever medicines such as Ibutabs and Ibusal, heartburn treatment Antepsin, cold remedy Nasolin, and cough suppressant Codetabs. Some products are expected to remain unavailable into late 2025 or beyond.
Prescription shortages affect treatments for a wide range of conditions, including anxiety, HIV, cancer, high blood pressure, asthma, migraine, epilepsy, and infertility. Notable products currently unavailable include Diapam, Yaz contraceptive pills, and the strong painkiller Fentanyl, supplied in various forms.
Panacod, another widely used pain medication, is missing from shelves in all its package sizes, 20, 50, and 100 tablets, until at least the end of September.
In one of the longest-listed disruptions, the adrenaline-based injection Emerade, used for emergency treatment of life-threatening allergic reactions, is not expected to return to full availability until the end of 2027. Fimea notes that alternative products and treatments are available and that manufacturers provide all duration estimates.
Over 80 medicines are expected to remain in shortage through 2026.
According to Fimea, most shortages are short-term. Some may resolve before mid-September, but others will persist due to production challenges, raw material shortages, or unexpected demand spikes. Capacity issues at international pharmaceutical plants are a major contributing factor, as many manufacturers serve global markets.
The availability of medicines at individual pharmacies can be checked by asking the pharmacy directly or by using pharmacy medicine search tools and the University Pharmacy’s website or online store.
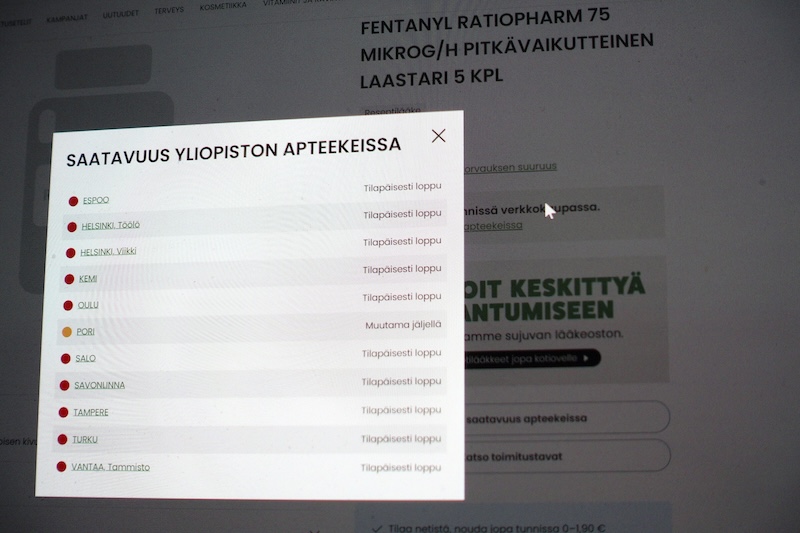
Availability status of Fentanyl Ratiopharm extended-release patches, as shown on the University Pharmacy’s website. Photo: Teemu Salonen / Lehtikuva
Although the situation is affecting Finland, it is not unique to the country. The agency stressed that medicine suppliers are responsible for maintaining stock, informing Fimea of shortages, and updating availability information.
Fimea publishes the supply disruption data based on reports submitted by pharmaceutical companies.
HT