 Credit: DR P. MARAZZI/SCIENCE PHOTO LIBRARY/Getty Images
Credit: DR P. MARAZZI/SCIENCE PHOTO LIBRARY/Getty Images
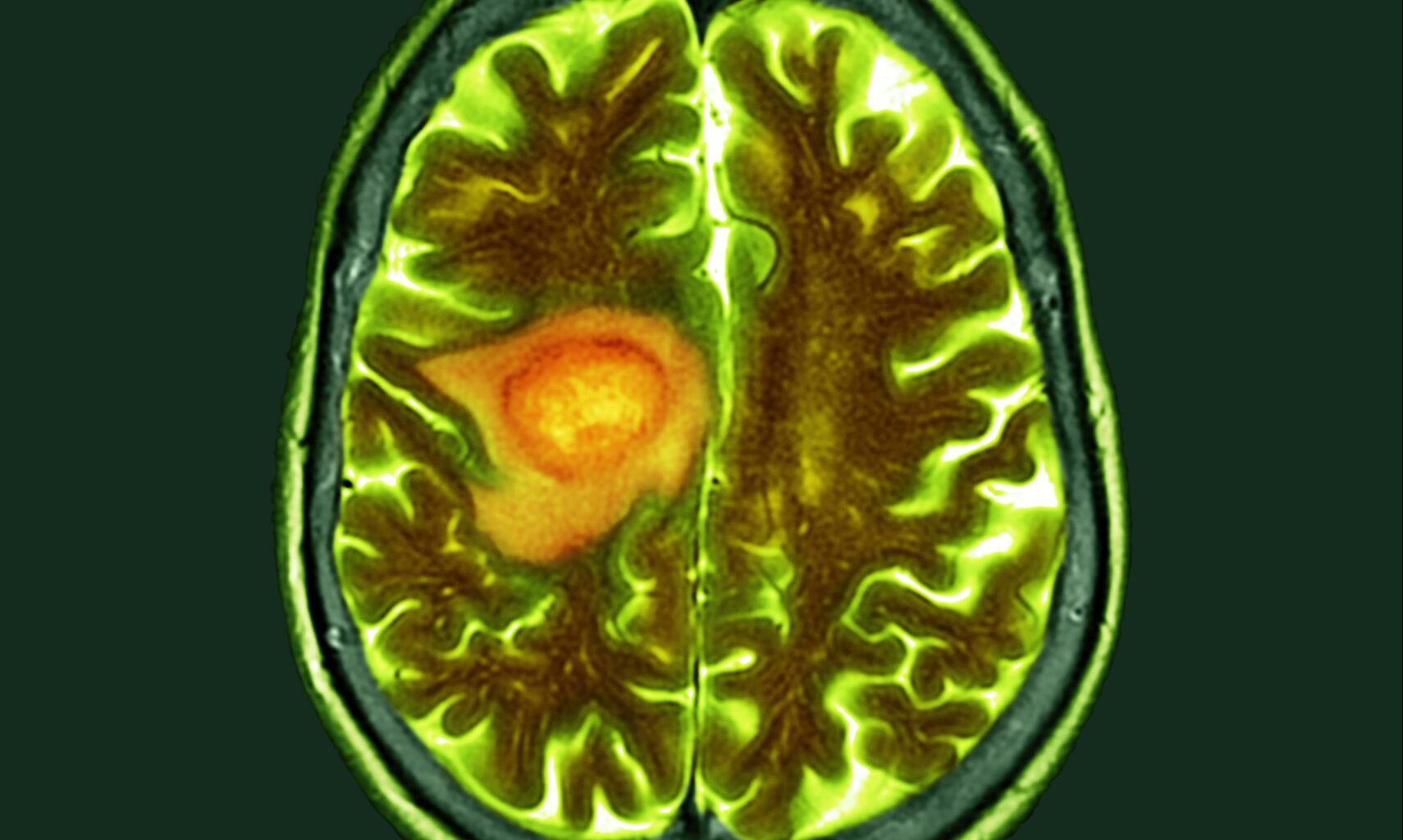
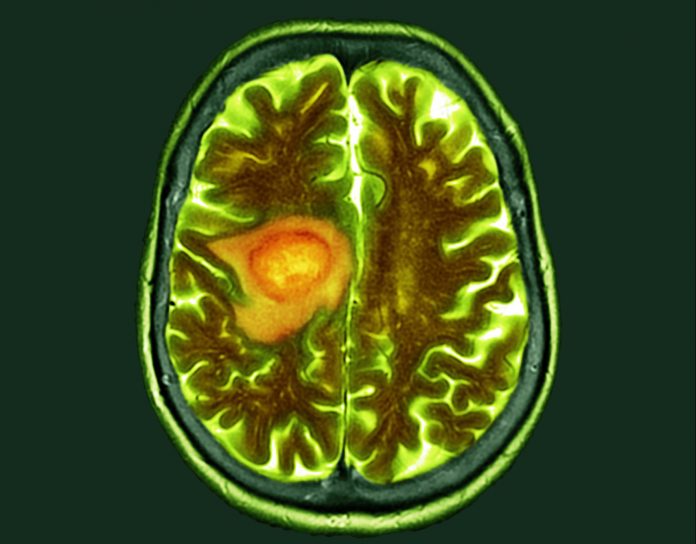
Researchers at Uppsala University in Sweden, in collaboration with scientists at the Queen Mary University of London, U.K., and the Dana-Farber Institute in Boston, Massachusetts, U.S., have discovered new mechanisms by which glioblastoma infiltrates the brain and identified key genes that drive its spread.
As the most common and aggressive type of brain tumor, glioblastoma accounts for almost 48% of all tumors of the central nervous system. While glioblastoma is rare, occurring in two to five per 100,000 people, it has a very poor prognosis. The survival rate in the first year after diagnosis lies at 40%, falling to 17% in the second year.
Glioblastoma is extremely difficult to treat. The high lethality is due to its fast-growing, aggressive nature, spreading invasively within the brain rather than forming metastases throughout the body.
“Invasion is now the defining challenge in glioblastoma,” Sven Nelander, PhD, Professor at the Department of Immunology, Genetics, and Pathology at Uppsala University and senior author of the new study, told Inside Precision Medicine.
“Patients used to die from the bulk tumor, but today the cancer spreads into vital regions like the brain stem, which we can’t remove or treat effectively. This is also a long-standing mystery—distinct invasion patterns were described as early as the 1940s, but we still don’t fully understand them.”
The international research team, led by Nelander, wanted to understand more about the invasion patterns of glioblastoma cells. They therefore studied exactly how glioblastoma cells spread in the brain, and specifically, how this spread is controlled. With this knowledge, new treatments might be developed, the researchers speculated.
In their study, published in the journal Nature Communications under the title “The invasion phenotypes of glioblastoma depend on plastic and reprogrammable cell states,” the scientists discovered that different glioblastoma tumor cells reveal varying growth patterns.
While some tumor cells spread diffusely throughout the brain tissue, others grow directly along blood vessels in the brain. How the cancerous cells will grow is determined by the tumor cell states, the researchers found.
The research team studied patient-derived mouse models and clinical tumor samples from patients with a combination of single-cell profiling and spatial protein detection. They found a close correlation between the state of differentiation in glioblastoma cells and their invasion routes in the brain.
Using computational modeling, the researchers also discovered the genes responsible for the cells’ different invasion routes. The gene ANXA1 drives tumor cells to grow along blood vessels, while the genes RFX4 and HOPX are active in glioblastoma cells that invade the brain tissue diffusely.
By knocking out the genes in mouse models, the researchers observed a change in the invasion routes of glioblastoma cells. When ANXA1 was knocked out, the glioblastoma cells completely stopped growing along the blood vessels, while ablation of HOPX and RFX4 did not change the phenotype, but led to a change in cell states, and even extended the survival in some mice.
The researchers then took a closer look at the tumor samples from glioblastoma patients and were able to detect the presence of the proteins encoded by the ANXA1, RFX4, and HOPX genes.
The presence of the proteins ANXA1, also known as annexin A1, and RFX4, also called regulatory factor X4, specifically, was associated with poor survival. This, say the researchers, could indicate these proteins as valuable prognostic biomarkers.
Nelander explained further: “The clearest and most exciting discovery is a new protein marker, ANXA1, which we linked to perivascular invasion—the way tumor cells use blood vessels as highways to spread through the brain. Blocking ANXA1 disrupts this process, which could open new ways to stop the tumor from spreading.”
The researchers believe that, in the future, the specific invasive cell states could be targeted as a therapeutic strategy in glioblastoma.
“Our results point directly to new strategies that target invasion rather than just the bulk tumor,” said Nelander. “We are already testing drug screens and genetic screens to find ways to attack these invasive cell states, and we’ve seen promising early hits.”
“We’re building new experimental systems—brain slices and organoids—where we use AI to track individual cells and predict treatments that could block invasion. It’s a very exciting way to connect biology and technology to find new therapies,” Nelander added.