Study design and population
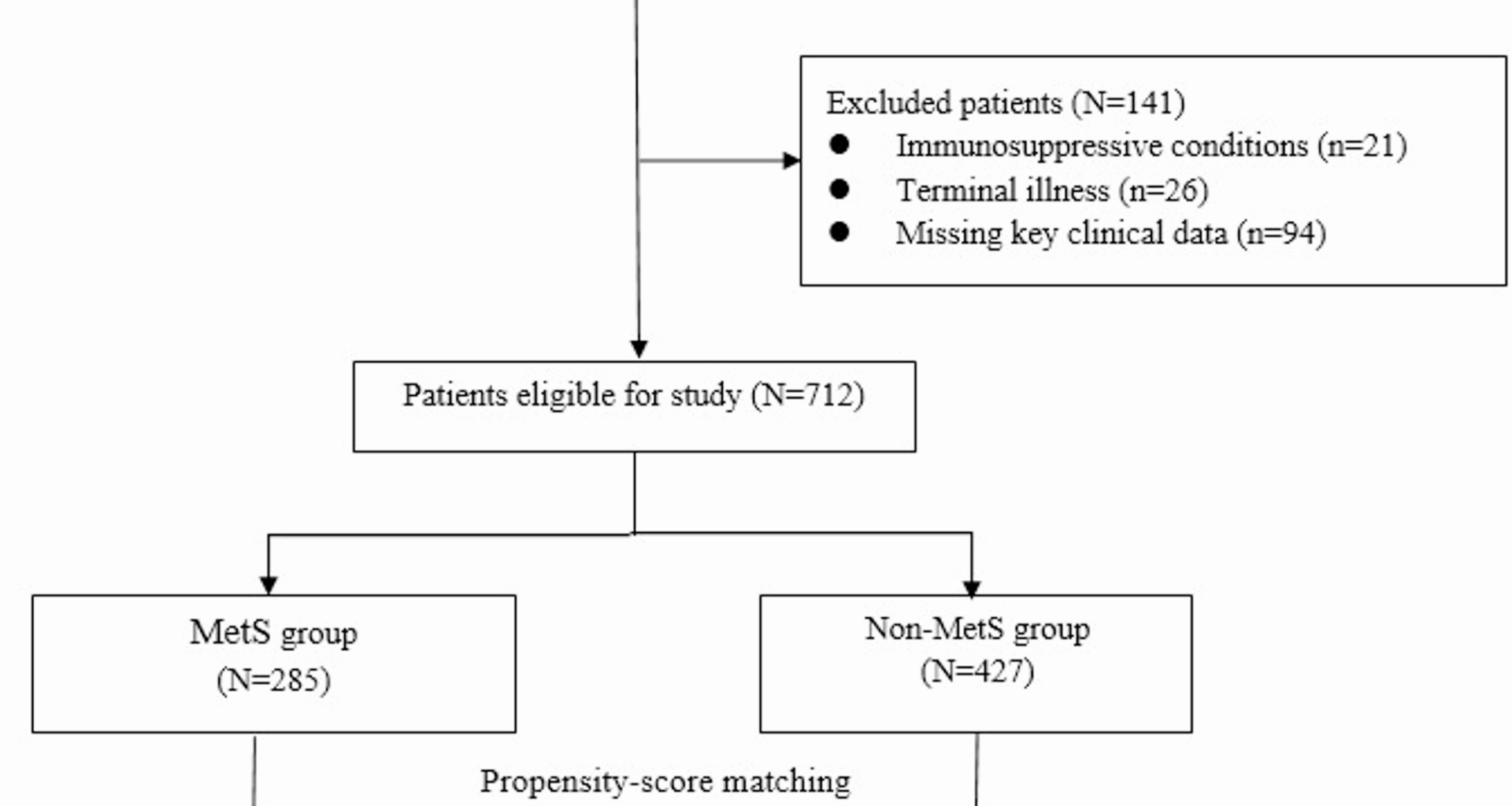
This retrospective cohort study was conducted at Huanggang Central Hospital, a tertiary care hospital. We included elderly patients aged ≥ 65 years who were admitted with a primary diagnosis of CAP between January 1, 2018, and December 31, 2022. CAP was diagnosed according to standardized clinical, radiological, and microbiological criteria, including the presence of new pulmonary infiltrates on chest imaging, in combination with symptoms such as cough, sputum production, fever, or dyspnea, and no hospitalization within the prior 14 days. All diagnoses were retrospectively confirmed by two attending physicians, who independently reviewed medical records, radiographic reports, and laboratory data. Discrepancies were resolved by consensus, and standardized protocols were followed to ensure consistency. Patients were excluded if they had: (1) hospital-acquired pneumonia, (2) immunosuppressive conditions (e.g., HIV/AIDS, organ transplantation, chemotherapy within 6 months), (3) missing key clinical data, or (4) terminal illness with an expected survival of < 30 days due to non-pulmonary causes.
Definition of metabolic syndrome
Central obesity (waist circumference ≥ 90 cm in male or ≥ 80 cm in female) is one of the diagnostic criteria of MetS outlined by the Chinese Diabetes Society (CDS) [9]. The International Diabetes Federation definition suggests that If BMI is > 30 kg/m², central obesity can be assumed and waist circumference does not need to be measured [10]. In this study we used Asian cutoff for obesity that is, ≥ 25 kg/m2 11. The MetS was defined as the presence of 3 of the following 5 criteria according to previous studies [11, 12]: (1) Obesity: BMI ≥ 25 kg/m2; (2) Hyperglycemia: FPG ≥ 6.1 mmol/L or 2-h PG ≥ 7.8 mmol/L, or known diabetes; (3) Hypertension: BP ≥ 130/85 mmHg or on treatment; (4) TG ≥ 1.7 mmol/L; (5) HDL-C < 1.04 mmol/L. Based on these criteria, patients were stratified into two groups: those with MetS and those without, at the time of CAP diagnosis.
Data collection and outcomes
Demographic data, comorbidities, chest X-ray, laboratory results, pathogens, and pneumonia severity index (PSI) scores were extracted from electronic medical records.
The primary outcome was 90-day all-cause mortality rate, with survival data obtained from medical records or, when necessary, through telephone follow-ups. It was selected based on its widespread use in pneumonia outcome research and its relevance to capturing both short-term complications and post-discharge mortality risks in elderly patients. This timeframe allows for assessment of the cumulative impact of metabolic dysregulation on recovery and survival. Secondary outcomes, including 30-day mortality and hospital length of stay, were chosen to reflect early clinical deterioration and healthcare burden, respectively. These outcomes were pre-specified based on clinical relevance and previous studies on pneumonia and metabolic comorbidities [13, 14].
Statistical analysis
The overall completeness of the dataset was high, with missing data < 5% for all variables. Missing data for covariates were handled using multiple imputations with chained equations (MICE), under the assumption of missing at random (MAR).
To minimize potential bias arising from baseline differences between the MetS and non-MetS groups, propensity score matching (PSM) was applied. Propensity scores were estimated through logistic regression, incorporating covariates such as demographic data, comorbidities, chest X-ray, laboratory results, pathogens, and PSI scores. A 1:1 matching was conducted between MetS and non-MetS patients using a nearest-neighbor algorithm without replacement, with a caliper width set to 0.2 times the standard deviation of the logit of the propensity score. To assess the balance of covariates after matching, standardized mean differences (SMDs) were calculated for all baseline characteristics.
After PSM, 336 patients (168 matched pairs) were included in the final analysis. Assuming that about 20% of participants would die by the time of the primary analysis (with a two-sided type I error rate of 5%) and a 10% non-response and attrition rate, a power of > 90% was provided to detect a hazard ratio of 2.0. This HR was chosen based on our exploratory data and supported by findings from a previous study by Liu et al. [15] which reported a similar HR for mortality risk in elderly pneumonia patients with diabetes mellitus.
Continuous variables were expressed as mean ± SD or median (IQR) and compared using Student’s t-tests or Mann-Whitney U tests, as appropriate. Categorical variables were compared using Chi-square or Fisher’s exact tests. Kaplan-Meier curve and multivariate Cox proportional hazards regression was used to analyze time-to-event data (90-day mortality). The proportional hazards assumption was assessed using time-dependent covariates and log(-log[survival]) plots, which confirmed that the assumption was not violated. To enhance the robustness of findings, the subgroup analyses were performed to evaluate the association of MetS status with 90-day mortality in patients stratified by age, gender and PSI. A p value < 0.05 was considered statistically significant. This study was conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. A completed STROBE checklist has been provided as a supplementary Table 1.
All statistical analyses were performed with the SPSS statistical software package (SPSS version 22.0 for Windows, Armonk, NY: IBM Corp.).