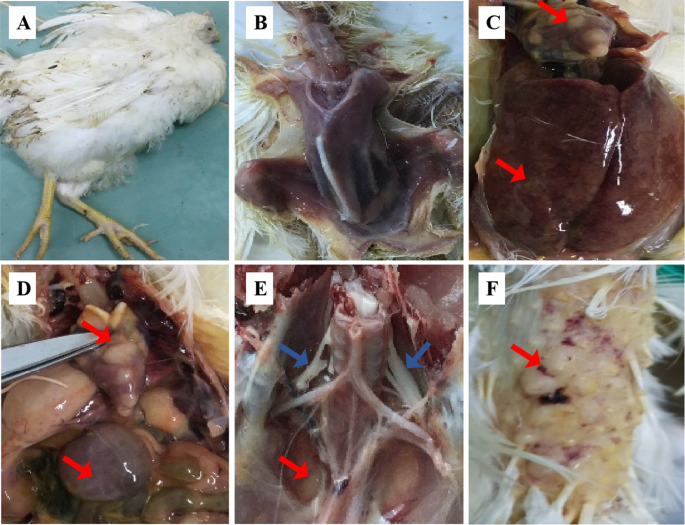
Clinical symptoms and PCR analysis samples detection of suspected MDV-infected chickens
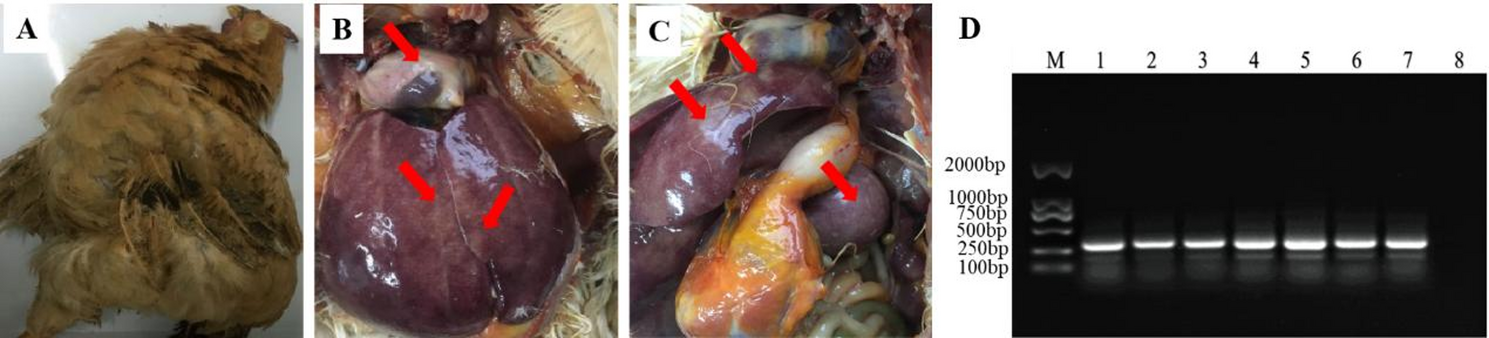
A serious outbreak of MD has occurred at poultry farm vaccinated with CVI988/Rispens in Guangdong, China. The diseased Qingyuan Partridge chickens showed symptoms of death, paralysis, emaciation, with the mortality rate of 18.6% and up to 40% of tumor in suspected chickens. Dissection of sick chickens revealed liver, spleen, and heart with tumor nodules, and the enlargement of the spleen and liver (Fig. 1). PCR analysis results showed that the MDV isolate contained two 132 bp repeats (bpr) in the genome, which was the same as the number of 132 bpr in the genome of very virulent positive control Md5 strain, indicating this isolate was a wild MDV isolate.
Gross clinical symptoms and PCR analysis of MDV 132bpr
(A)The bird showed stunted growth and paralysis. (B-C) Dissection of sick chickens showed enlarged liver and spleen with tumor nodules in the heart, liver, and spleen(red arrow). (D) PCR detection of MDV 132bpr, Lane M: DL 2000 DNA marker; Lane 1–6: tumor tissue DNA from MD chickens; Lane 7: the very virulent MDV positive control Md5 strain with two copies of the 132-bpr; lane 8: negative control, CEF culture without infection.
Isolation and identification of SS1901 strain
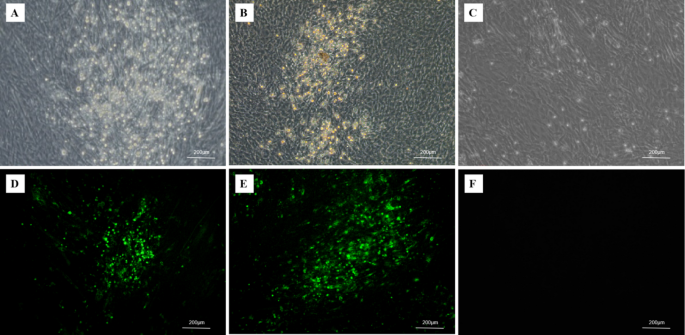
The isolated lymphocytes collected from MDV-infected birds were inoculated on CEF monolayer, then incubated at 37◦C with 5% CO2 for 7 days until the typical CPEs were observed. After plaque purification, SS1901 strain was isolated and the infected CEF displayed rounded, aggregation with enhanced refractive changes, which were similar with the hypervirulent Md5 strain infection (Fig. 2A-C). IFA was carried out with mAb GB2 specific for the serotype 1 MDV strains, showing green fluorescence signals in cells with obvious CPEs, but not in uninfected CEFs (Fig. 2D-F). The assessment of adventitious agents for ALV, REV, and CIAV was all negative.
The SS1901 strain infected CEF produced typical plaques and were detected by IFA. (A) Plaques were formed by the SS1901 strain infection; (B) Plaques were formed by the Md5 strain infection; (C) CEF; (D) Viral plaques of SS1901-infection were detected with MDV gB-specific monoclonal antibody using IFA. (E) Viral plaques of Md5-infection were detected by IFA; (F) CEF as negative control
Sequencing and phylogenetic analysis of Meq gene
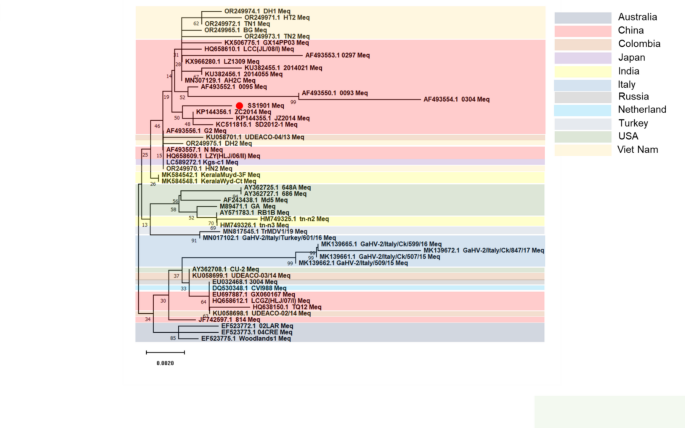
Sequencing analysis of the full Meq gene of the SS1901 strain showed the gene is 1020 bp long with 339 codons (Accession numbers: PP883553.1), and its similarity of with 52 reference strains was ranged from 98 to 99.87%. Generation of the evolutionary tree by MEGA.11 based on the genetic distance matrix with the neighbor-joining method (Fig. 3), revealed that SS1901 strain belongs to a similar branch of the highly virulent strains isolated obtained in China. The Meq gene of the SS1901 isolate exhibited the highest homology with those found in the ZC2014, LZ1309 and AH2C strains, isolated from Shandong, Gansu and Heilongjiang Provinces, China, respectively. It shares 98.7% homology to vv + MDV 648 A and 686 strains, 99.0% homology to vvMDV Md5 and RB1B strains, and 98%, the lowest homology homologous to the isolate GaHV-2/Italy/Ck/847/17 from Italy (Fig. 3). This gene shared 98.7% and 98.8% sequence identity with the mMDV CVI988 and 814 vaccine strains (Fig. 3). These findings demonstrate that the SS1901 isolate is most likely a newly emerging highly pathogenic wild strain.
Phylogenetic trees based on the Meq nucleotide sequences of MDV
In the 339 amino acids predicted to be encoded by the Meq gene of SS1901 strain, several conserved amino acid mutation sites were found to be same as those in other Chinese isolates, including at positions K77E, D80Y, V115A, T139A, P176R, and P217A (Table 2). Comparing with 52 reference sequences, the SS1901 strain showed an A-to-T mutation at position 88. This mutation was also found in Chinese isolates JZ2014, SD2012-1, and ZC2014, but not has not in isolates from other countries or regions. An additional Q-to-R mutation at position 93 is also present in Italy isolates Italy/509/15, Italy/Ck/507/15, Italy/Ck/599/16, and Italy/Ck/847/17 (Table 2). As a significant number of recent isolates of wild-type MDV in Southern China, also possess the A88T and Q93R mutations, it indicates that strains with these two mutations are becoming prevalent in circulating wild-type MDV strains.
Table 2 Amino acid mutations in the Meq gene of isolates from Southern ChinaSurvival curves of SPF chickens infected with SS1901
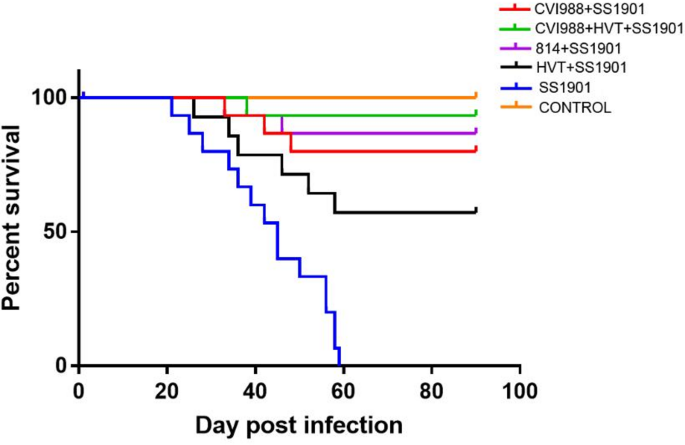
To evaluate the pathogenicity of SS1901 isolate, a total of 120 one-day-old SPF chickens were randomly divided into six groups. In the SS1901 infection group with 20 chickens in each group. In the SS1901-infected group, the earliest death cases occurred at 21 days post infection, followed by the peak of mortality at 30–60 dpi with nearly 100% death at 60 dpi (Fig. 4). The mortality rate in the HVT vaccine-immunized group was 40% at 90 dpi, with the earliest death occurring 26 dpi (Fig. 4). The 814 and CVI988 immunized groups experienced the earliest deaths at 33 dpi, with the morbidity of 13.3% and 20% at 90 dpi, respectively. The CVI988 + HVT immunized group saw a mortality rate of 6.67%, and the earliest death happened at 38 dpi (Fig. 4). These results demonstrated that the SS1901 isolate was a highly lethal strain, and the HVT vaccine could no longer provide protection, CVI988, 814 and even CVI988 + HVT did not provide complete protection.
Survival curve of the experimental groups
Clinical symptoms, tumorigenicity and vaccine protection index of SS1901 isolate infection in SPF chickens
Chicken infected with SS1901 isolate showed depression, ataxia and paralysis at 20 dpi(Fig. 5A-B). Dissection of these infected chickens showed hepatomegaly and splenomegaly with white tumor spots in livers) and spleens (Fig. 5C-D). Most infected chickens had visible tumor nodules in their hearts (Fig. 5C-D). Some infected chickens showed enlarged kidneys with white necrotic spots and white tumor nodules in the gonads (Fig. 5E). There were also lesions of tumor and sciatic nerve enlargement on the leg skin of the affected chickens (Fig. 5F). The results indicated that SS1901 infection could cause typical clinical symptoms of very virulent MDV strains, with death, paralysis, visceral tumors, and skin tumors.
The clinical manifestations of SS1901 isolate infection in SPF chickens. (A) Paralysis in SPF chickens infected with SS1901 isolate; (B) Extreme emaciation in SPF chickens infected with SS1901 isolate; (C) Hepatomegaly with tumors in liver and heart; (D)splenomegaly with tumors in spleen and heart; (E) Sciatic nerve enlargement and tumors in kidneys; (F) Tumors on the skin of the legs. Red arrows indicate tumor nodules and blue arrows indicate sciatic nerve
In terms of tumorigenesis, no chickens in the control group developed tumors. Nevertheless, chickens in the SS1901 infection group developed MD symptoms with a tumorigenicity rate of 80% (12/15) (Table 3). The majority of the tumors in this group were found in the heart (12/15), liver (8/15), and spleen (11/15), and also appeared in the kidneys and glandular stomachs (6/15), while three infected chickens developed skin-type tumors (Table 3). Chickens in the HVT-vaccinated group exhibited a tumor incidence of 40%, with tumors predominantly involving the heart (6/15), followed in the liver, spleen, and glandular stomach (2/15), while kidney and skin tumors appeared in 1 case. Following the vaccinated groups with CVI988 + HVT, CVI988 and 814, the incidence rate of MD was 13.3%, 26.7%, 20%, tumorigenicity was 13.3%, 20%, and 20%, and vaccination protection index was 86.7%, 73.3%, and 80%, respectively (Table 4). These results confirmed the high pathogenicity of SS1901, characterized by visceral tumor formation in critical organs. Furthermore, the findings suggested that vaccination could reduce the incidence of tumor development associated with MD, but it does not completely eliminate the risk.
Table 3 Tumor incidence and distribution in chickens of experimental groupsTable 4 Morbidity, mortality and vaccine protection in experimental groupsEffect of MDV SS1901 infection on body weight and immune organ index
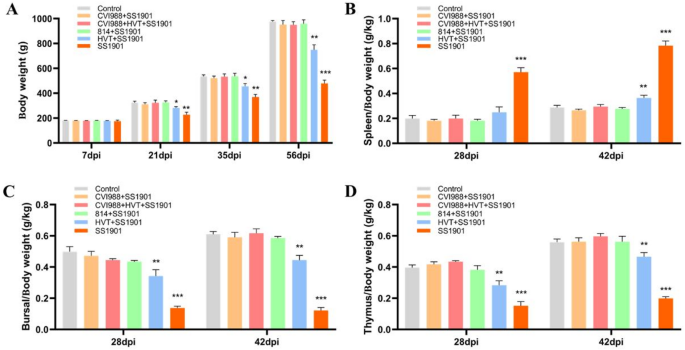
Five chickens infected with SS1901 were randomly selected from each group and weighed at 7, 21, 35, and 56 dpi. Meanwhile, three chickens from each group were randomly chosen at 28 dpi and 42 dpi, respectively, for weighing the spleens, bursa, and thymus to calculate immune organ indices. No significant difference in the rate of body weight gain between the control and immunized groups at 7dpi (Fig. 6A). However, the body weight of the SS1901-infected group was significantly lower than the control group at 21dpi and 35 dpi (p < 0.01) (Fig. 6A). At 56 dpi, chickens in the SS1901 group exhibited severe emaciation, with a markedly reduced mean body weight compared to the control group (p < 0.001) (Fig. 6A). Additionally, the HVT immunized group also showed a significant reduction in body weight relative to the controls (p < 0.01) (Fig. 6A). Furthermore, the spleens of chickens in the SS1901-infected group were significantly enlarged compared to the control group (p < 0.001), while the bursa and thymus were significantly atrophied at 28 dpi and 42dpi(p < 0.001), with the spleen index substantially higher than that in both the vaccinated and control groups (p < 0.001) (Fig. 6B). Conversely, the bursa and thymus indices were significantly lower, showing a pronounced difference compared to the immunized and control groups (p < 0.001) (Fig. 6C-D). Overall, these findings demonstrated that SS1901 infection significantly reduced the weight gain and severely impaired the development of immune organs in SPF chickens, thereby compromising the immune function.
The effect of SS1901 infection on body weight and immune organ index in SPF chickens (A) Effect of different days post SS1901 infection on body weight(g) of SPF chickens; (B) Organ index of the spleen(g/kg); (C) Organ index of the Bursa(g/kg); (D) Organ index of the thymus. The data are shown as the mean ± SD and differences were considered statistically significant at p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***)
Histological examination and PCR detection of tumor tissues in SPF chickens infected with SS1901 strain
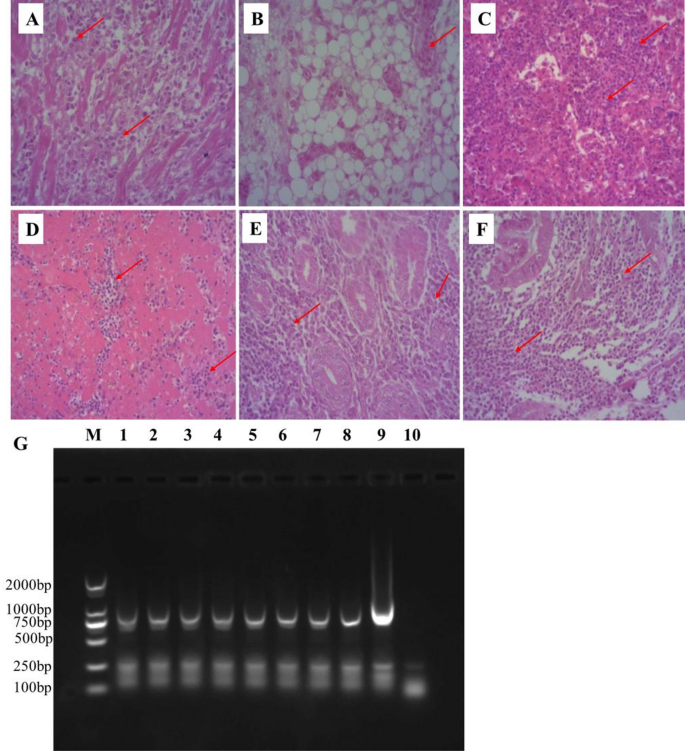
Organs and tissues collected from chickens challenged with SS1901 isolate were examined by histopathological staining (H&E) and PCR. Histopathological examination of H&E stained heart, liver, spleen, kidney, proventriculus, and bursa of fabricius tissues from SS1901 infected birds revealed distinct pathological features (Fig. 7). Cardiac tissue sections demonstrated focal lymphocyte infiltration with interstitial lymphocyte proliferation between myocardial fibers, accompanied by vascular tumor cell distribution within blood vessels (Fig. 7A-B). Hepatic sections exhibited hepatocellular degeneration and necrosis, characterized by diffuse lymphocyte infiltration extending through hepatic sinusoids and interlobular areas (Fig. 7C). Splenic architecture showed structural disintegration with endothelial cell exposure, indistinct demarcation between red pulp and white pulp, and white pulp atrophy (Fig. 7D). Notably, multifocal lymphocyte aggregations of varying sizes formed distinct infiltrative foci (Fig. 7D). Renal pathology manifested as interstitial expansion in tumor-affected regions, accompanied by diffuse lymphocyte proliferation within the renal interstitium (Fig. 7E). Proventricular sections revealed polymorphic lymphocyte aggregation with focal proliferation patterns and observable tumor cell mitotic figures (Fig. 7F). PCR amplification was performed on tissue samples collected from infected chickens with SS1901 strain, including the liver, spleen, bursa of fabricius, heart, proventriculus, kidney, and feather pulp, using primers specific to the Meq gene. The results indicated a target amplicon of approximately 1020 bp in all samples, corresponding to the size of the Meq gene in the Md5 reference strain (Fig. 7G). This finding serves as a molecular indicator confirming that the infected chickens developed disease due to SS1901 infection. Collectively, these findings demonstrate that infection with the MDV isolate SS1901 in-duces characteristic visceral tumors in heart, liver, spleen, kidney and glandular gastric.
Histopathological observation of tissues in SPF chickens infected with SS1901 isolate (A) Heart tissue sections showing lymphocyte hyperplasia (H.E. staining, 200×); (B) Distribution of hemangioma cells in cardiac vascular tissue sections (H.E. staining, 200 ×); (C) Liver tissue sections exhibited extensive lymphocytic infiltration (H.E. staining, 200×) (D) Spleen tissue sections with significant lymphocyte aggregates (H.E. staining, 200×); (E) Kidney tissue sections showing Lymphocytes proliferation in the renal interstitium (H.E. staining, 200 ×);(F) Glandular gastric tissue sections showing polymorphic lymphocyte aggregation (H.E. staining, 200 ×). (G) PCR detection of Meq gene, Lane M: DL 2000 DNA marker; Lane 1–7: The tissues DNA extracted from of SS1901-infected chickens: liver, spleen, bursa of fabricius, heart, proventriculus, kidney, and feather pulp, respectively; Lane 8: DNA from CEF culture with SS1901 strain; Lane 9: positive control of vvMDV Md5 strain; Lane 10: negative control, CEF culture without infection. Red arrows indicate polymorphic lymphoproliferative cells