Patients with aggressive leukaemia have been offered hope of a cure by a “living drug” that is being rolled out on the NHS.
The treatment involves genetically modifying the patient’s own immune cells in the lab, then returning them to the body where they attack and kill cancer cells in the bloodstream.
The drug, called obe-cel, was developed in the UK by Autolus, a spinout company of University College London, and has shown “wondrous” results in trials.
Researchers said the treatment would “save many lives”, with 77 per cent of patients entering remission after treatment. Currently patients with leukaemia live for ten months on average after the standard chemotherapy treatment.
Nice, the health watchdog, has approved obe-cel for use on a specific sub-type of leukaemia in specialist NHS centres. About 50 patients a year with B-cell acute lymphoblastic leukaemia are expected to benefit, receiving two doses ten days apart.
Obe-cel is a type of immunotherapy called Car T-cell therapy, which works by harnessing the body’s own immune system to attack cancer cells.
Scientists take a sample of T-cells, an immune cell that fights infection, from a patient’s blood. These are then genetically engineered in the lab into so-called Car T-cells, which recognise a specific protein on the surface of cancer cells.

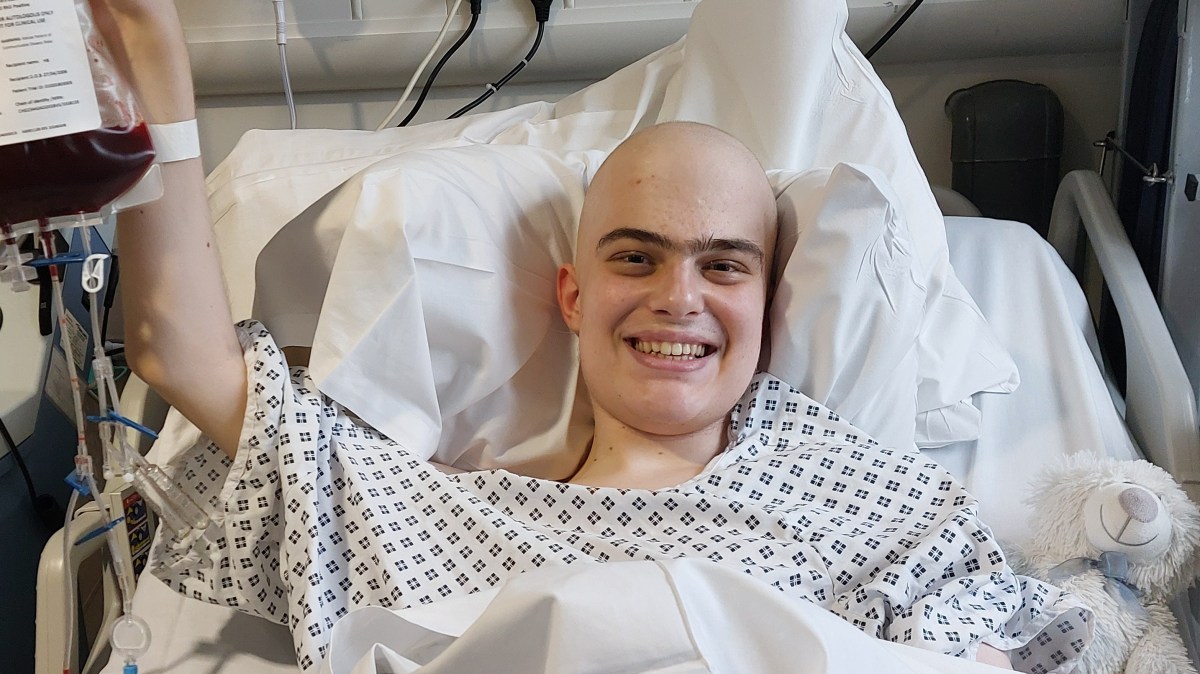
Harry Brown, 19, with his parents, is in remission after he took part in the trial
Professor Peter Johnson, NHS national clinical director for cancer, said: “This cutting-edge therapy has shown real promise in trials and could give patients with this aggressive form of leukaemia a chance to live free from cancer for longer. For some, it could offer the hope of a cure.
“This ‘living medicine’ boosts a patient’s own immune system and then guides T-cells towards the cancer to kill it. It is fantastic to have another pioneering option available on the NHS, adding to our range of Car-T therapies, which are helping people with blood cancers live longer, healthier lives.”
Of the 77 per cent of patients whose cancer entered remission, half are still showing no signs of detectable cancer after three and a half years. On average, the treatment gave patients 15.6 months additional months of life.
Patients to have benefited include Harry Brown, a 19-year-old student from Harrogate, who was treated with obe-cel as part of a clinical trial in 2024. He said: “I feel so lucky to have had access to such a wondrous treatment. Not only did it work better than my doctors thought it would, it worked without many of the horrible side-effects you can get from other treatments.

Brown said there were no side-effects from the treatment
“The biggest thing it offers is hope. When you’re facing a situation like mine, hope is the most valuable thing you can have.”
Fiona Bride, the interim chief commercial officer at NHS England, said: “This is a success story that’s made in Britain, and shows how collaboratively we can bring to life the ambition of the 10 Year Health Plan, showcasing how the UK’s competitive edge in life sciences can translate to better outcomes and treatments for NHS patients.
“The journey of obe-cel from scientific research in a UK university to a safe, clinically and cost-effective treatment set to be delivered through the NHS specialist Car-T network is a remarkable one and I am grateful to colleagues who have played their part along the way.”