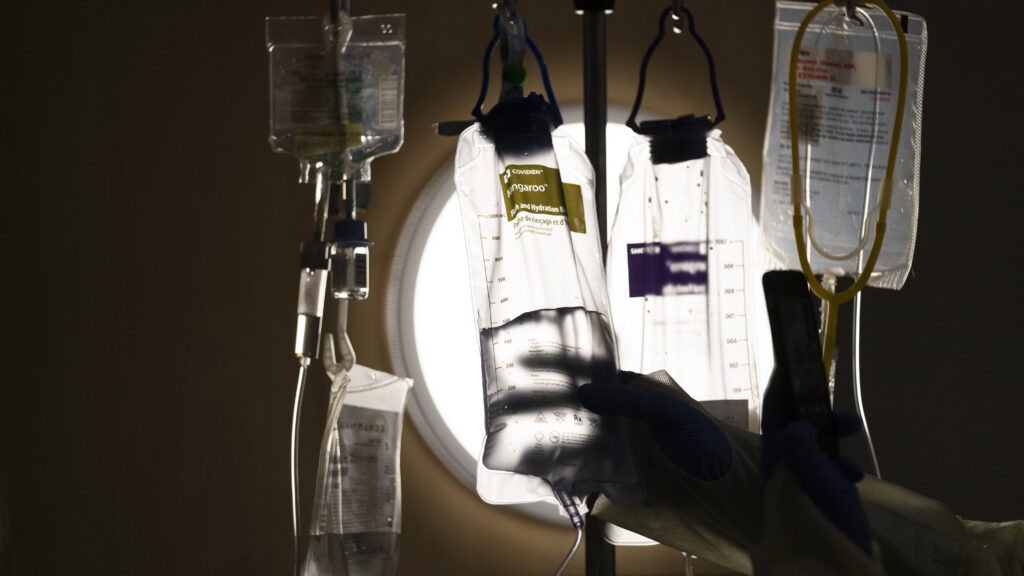
In a startling failure of quality control, Amneal Pharmaceuticals relied on contaminated bags for a sterile injectable drug even after identifying the risk and then lowered its standards so that the bags could continue to be used, according to an Aug. 27 warning letter issued by the U.S. Food and Drug Administration.
The problem was identified during an agency inspection last March at a facility in India run by the company, which is a large supplier of generic and specialty medicines. At the time, FDA inspectors noted that employees failed to investigate the presence of white polypropylene fibers that were found in bags used to hold ropivacaine hydrochloride, which is injected into the epidural space.
The agency complained that Amneal “minimized the severity” of the issue in different ways. For one, it said, the company was aware of the risk of particle contamination from the polypropylene bags, which were purchased from a supplier, for more than a year before an internal investigation was conducted in 2024. In fact, Amneal had rejected several batches after investigating the problem in late 2022 and early 2023.
STAT+ Exclusive Story
Already have an account? Log in
This article is exclusive to STAT+ subscribers
Unlock this article — plus in-depth analysis, newsletters, premium events, and news alerts.
Already have an account? Log in
Individual plans
Group plans
To read the rest of this story subscribe to STAT+.