Professor Stefanie Dehnen, Executive Director of the Institute of Nanotechnology at the Karlsruhe Institute of Technology (KIT), discusses how the BiCMat project is unlocking new frontiers in materials chemistry through the exploration of bismuth cluster compounds.
Bismuth is a unique element that has so far been largely overlooked in research compared to other metals or semimetals in the periodic table. However, its unusual properties and chemical versatility offer untapped potential for the development of next-generation materials.
Led by Professor Stefanie Dehnen at the Karlsruhe Institute of Technology (KIT), the BiCMat project is working to expand our knowledge of bismuth and use bismuth-based clusters to create entirely new materials. Supported by an ERC Advanced Grant, the BiCMat project aims to discover, isolate, and understand novel bismuth clusters, offering a window into unexplored molecular architectures and materials based upon them.
The Innovation Platform spoke to Professor Dehnen to learn how her team is using fundamental chemistry to open new doors in materials science.
Can you outline the BiCMat project and your key goals?
The BiCMat project focuses on bismuth cluster-based materials. We begin with a synthesis of molecular clusters (‘zero-dimensional’, 0D) and use them to not only advance our knowledge of these nanosized architectures, but also to take them to higher-dimensionality materials. We want to know what these materials can do for us. For example, when structured in a certain way, could these compounds be capable of hosting or separating gases and eventually acting as catalysts for small molecule activation? Or could they store lithium or sodium ions and then act as a battery material?
We also use insights from quantum chemistry and machine learning to make the synthesis more straightforward and optimise the results to be as useful as possible. To ensure we discover all that we need to know about these compounds, we utilise experimental methods such as spectroscopy and diffraction together with the theoretical tools, before taking the next step towards the material properties.
What makes bismuth different, and what potential does it have for new materials?
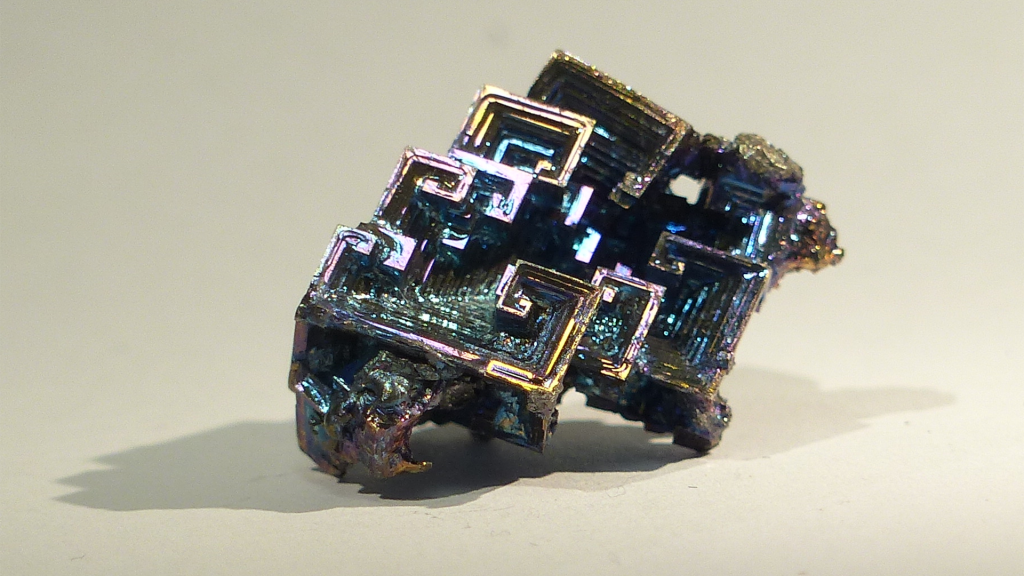
Bismuth is a very special element. It is considered a metalloid because, whilst its conductivity is fairly high, it is more in the range of semiconductors. Its structure is analogous to the structures of arsenic and antimony and related to that of black phosphorus. The structure shows directionality, rather than that of a typical metal with its dense packing of atoms bearing more isotropic interactions. Bismuth is a very heavy element – it is the heaviest (quasi-)stable element. It also displays particularities in its chemical reactions.
What makes bismuth particularly special is that it is completely non-toxic, which is especially interesting when looking for new materials. All of its neighbouring elements are really highly toxic, but for some reason, there is an island of non-toxicity, and this is why bismuth is now heavily investigated in all kinds of chemistries and physics, and even for medical applications. It has been used in organometallic chemistry and in purely inorganic chemistry – for example, for perovskite-like materials for photovoltaics. Now, our idea is to use that knowledge to include clusters into these materials and develop completely new, nanostructured, cluster-based materials.
As I mentioned previously, the potential to use the clusters as catalysts, for things like battery materials or for gas storage or separation, would offer much less harmful alternatives to many of the materials used today. We hope to achieve very functional new properties, but without the toxicity of some of the materials currently used.
There are also challenges in terms of handling bismuth, because our knowledge of it in molecular and materials chemistry so far is limited. Overcoming these challenges is a trial-and-error process involving a lot of experimental reactions. However, once you overcome them, you can achieve a new field of properties to make use of. Application of predictive theoretical tools further helps to streamline the process.
Your team is developing entirely new ways to connect these clusters into 1D, 2D, and 3D materials. Can you share a surprising property or breakthrough that has emerged from this approach?
When working from 0D up to other dimensionalities, one surprise involved a cluster that we reported based on its interesting structural and bonding features. Then, several years later, a different student achieved the same cluster, but as a pair of them connected by a bond. Cases like this highlight the large knowledge gap surrounding the formation processes that occur within the reaction solution and the need to learn more about them. One of the key parts of the project is to monitor the reactions and model them so that we can also design and control this instead of it being a surprise.
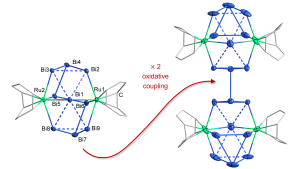
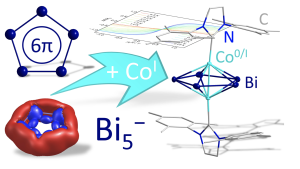
Another lesson learned was a cluster that is formed from three rings. If you consider a square of four bismuth atoms, three of them are connected by bismuth-bismuth bonds to form a ring. This very specific cluster triggered many questions, and we now understand a lot more about it. It is a so-called all-metal aromatic system, and it is the largest metal-π-aromatic system bearing the strongest ring current per electron, which again highlights the peculiarity of bismuth. By chance, this helped us jump into the field of metal aromaticity that we may make use of in the future. One more example of such species was recently realised upon systematic research with the capture of an aromatic five-membered ring made from bismuth atoms only. This was successful by using cobalt atoms to trap the ring, which helped us to report the first of its kind metal complex based on this uncommon metal-aromatic cycle.
Many of our structures surprise us at first, and then we investigate them and learn more about their formation. We look into the very early stages of the reactions to help us design the next steps.
Can you explain more about the progress with the machine learning side of the project?
At this stage, about halfway through the project, the machine learning is still at an early point in its development. We planned it to be much further developed so far, but it is a very time-consuming and computationally expensive process. At present, we are still feeding this process with data and knowledge from our site, but we are starting to see the first results evolving from this. Once the data-feeding process has come together, the output will rapidly increase. Within around 6-12 months, we hope to reach a point where we can learn from the data and achieve predictions of how to run the reactions more efficiently, for instance, by using the most appropriate reactants for a given target structure.
Where do you see the strongest real-world impact for these bismuth-based compounds?
One of the strongest impacts will be in the area of catalysis. We now know that even small units of bismuth can help in such activities, like the hydrogenation of olefins, for example. These compounds are also very variable in how they form, allowing us to design their activation properties very well.
We also want to gain more insight into their use as materials for photovoltaics and batteries, for example. Another potential application would be to use them for forming specifically structured and printed semiconducting devices.
If researchers or industry partners reading this article wanted to contribute or build on your discoveries, what collaboration or expertise would you most welcome as the project continues to grow?
We would welcome direction on how to create devices from these materials. Currently, we are collaborating with a colleague working on printing them. However, this is not currently in the project as it is a relatively new development. We hope to be able to take the materials – be it nanoparticles made from them or the clusters themselves – and fabricate working devices out of them.
The manufacturing of devices is not our area of expertise. For inkjet printing, for example, you need the compounds in a stable and printable ink. This requires a lot of expertise outside of cluster chemistry. An ink is a high-tech mixture and requires the right combination of solvents, stabilisers, etc. We therefore require direction from experts in this area about the appropriate ingredients, and we can then say how these will probably interact with our clusters and adapt this if needed. This can therefore only be done in collaboration.
We also need industry partners. At present, we can only work on a very small scale, and we need industry input to help scale up. In some cases, our reactions are very specific, and we therefore need direction from industries so we can understand what they need and how we can meet their requirements.
Please note, this article will also appear in the 23rd edition of our quarterly publication.