 Scientists have identified a previously unknown tubular structure inside a symbiotic bacterium that lives within the Asian citrus psyllid. Credit: Shutterstock
Scientists have identified a previously unknown tubular structure inside a symbiotic bacterium that lives within the Asian citrus psyllid. Credit: Shutterstock
The research is opening new frontiers in pest control and evolutionary biology.
An international group of scientists has uncovered a strange tubular structure within Profftella, a bacterium that lives in close partnership with a major citrus pest. Nothing like this structure has ever been documented in any living organism. The discovery, made possible through advanced microscopy, could open the door to new strategies for pest control and provide fresh insights into the evolution of life.
The research was carried out by scientists from Pusan National University, the National Institute for Physiological Sciences, Kobe University, and Toyohashi University of Technology. They identified the unusual structure inside Candidatus Profftella armatura, a symbiotic bacterium that inhabits the Asian citrus psyllid (Diaphorina citri). Their findings were published in NPJ Imaging on September 18, 2025.
The Asian citrus psyllid is a destructive insect that inflicts major damage on citrus crops around the globe, reducing harvests and driving up prices. This pest carries Profftella, a bacterium passed down through generations of psyllids. The microbe plays a crucial role in the insect’s survival and produces toxic substances that help shield its host from natural enemies.
Discovery of an Unprecedented Tubular Structure
Using advanced 3D electron microscopy, the team revealed that Profftella cells, which are themselves extremely elongated, measuring up to over 100 micrometers in length, contain multiple elongated tubular structures each measuring tens of micrometers in length. It was revealed that these tubes have a diameter of approximately 230 nm and are composed of 5 to 6 right-handed helical fibers forming a twisted structure. They were also found to occupy a consistent proportion of the Profftella cell volume.
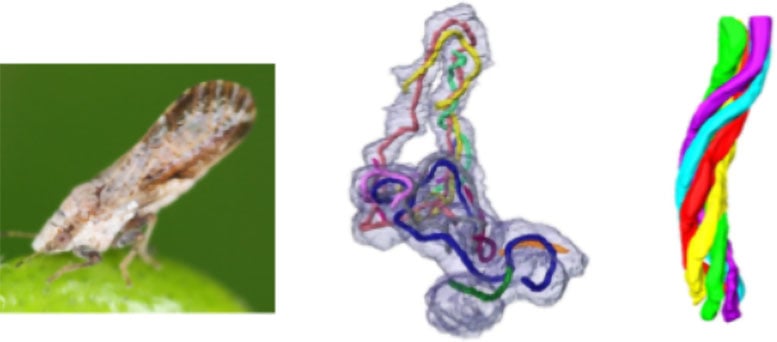 The Asian citrus psyllid (left), a representative elongated Profftella cell containing numerous intracellular tubules (middle), and the architecture of a single tubular structure (right). Credit: Toyohashi University of Technology
The Asian citrus psyllid (left), a representative elongated Profftella cell containing numerous intracellular tubules (middle), and the architecture of a single tubular structure (right). Credit: Toyohashi University of Technology
“Typically, bacteria lack such complex organelles. Moreover, I was surprised to find that this tube is so stable and robust that it maintains its shape during high-vacuum electron microscopy observation without chemical fixation or embedding,” explains Assistant Professor Chihong Song from Pusan National University, the article’s first author.
Further analyses using various techniques, including optical microscopy, revealed that these tubes contain numerous ribosomes, the cellular machinery responsible for protein synthesis.
“Based on this, the tubes may be involved in protein synthesis. Given their sturdiness, they could also provide physical support to the elongated Profftella cells, and maybe even act like a scaffold for material transport—kind of like the cytoskeleton in eukaryotic cells,” says Associate Professor Atsushi Nakabachi from Toyohashi University of Technology, a corresponding author of the study.
Rethinking Bacterial Complexity
The research team emphasizes that this structure represents an extremely rare example of an organelle found in bacteria.
This significant discovery challenges the conventional view of bacteria as simple life forms and offers new insights into the evolution of cellular structures. At the same time, it opens promising avenues for developing selective pest control strategies targeting the Asian citrus psyllid, with important implications for agriculture.
Reference: “Enigmatic tubular ultrastructure in the bacterial defensive symbiont of the Asian citrus psyllid Diaphorina citri” by Chihong Song, Junnosuke Maruyama, Kazuyoshi Murata, Toshinobu Suzaki and Atsushi Nakabachi, 18 September 2025, npj Imaging.
DOI: 10.1038/s44303-025-00107-w
This work was supported by the Japan Society for the Promotion of Science (https://www.jsps.go.jp) KAKENHI (grant numbers 21687020, 26292174, 20H02998 and 25K02023 to AN), the Collaborative Study by High Voltage Electron Microscopy Program (2015-502, 2016-502) of National Institute for Physiological Sciences to AN, and a National Research Foundation of Korea (NRF) grant RS-2024-00440289 to CS.
Never miss a breakthrough: Join the SciTechDaily newsletter.

