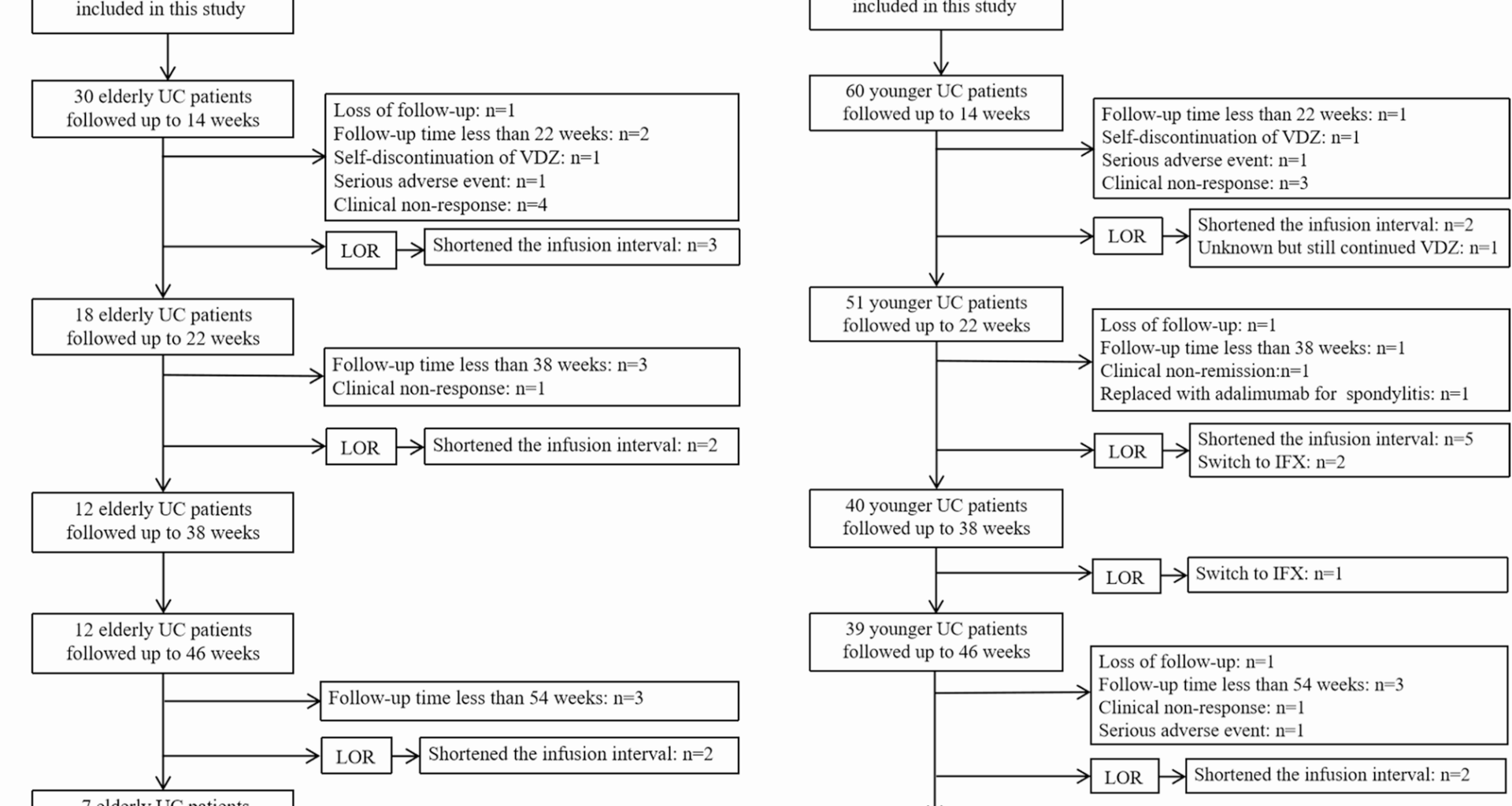
Study design and patients
We retrospectively enrolled patients with UC who were undergoing VDZ therapy at the Department of Gastroenterology, Affiliated Hospital of Qingdao University (Qingdao, Shandong Province, China) from March 2021 to November 2024. The inclusion criteria were: (i) A diagnosis of UC based on the combined assessment of symptomatology, endoscopy, abdominal imaging, and histological findings according to the European Crohn’s and Colitis Organisation guidelines [12], (ii) moderate-to-severe disease activity (modified Mayo score ≥ 6), (iii) age > 18 years at the time of the first VDZ dose, and (iv) having received at least three VDZ infusions [9]. The exclusion criteria were as follows: Crohn’s disease, age < 18 years, pregnancy or lactation, and undetermined disease activity or no colonoscopy before treatment.
Each patient aged ≥ 60 years at VDZ initiation was included in the elderly cohort and matched with two younger patients (aged 18–59 years). Younger patients were matched to elderly patients based on sex, disease duration, disease extent, disease activity, and Mayo endoscopy score (MES).
Patients were administered VDZ as standard induction and maintenance treatment (300 mg at weeks 0, 2, and 6, followed by every 8 weeks thereafter). During maintenance, should patients experience a loss of response (LOR), the interval between infusions shortened to every 4 (Q4W) or 6 (Q6W) weeks.
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki (World Medical Association, 2024). The Ethics Committee of the Affiliated Hospital of Qingdao University authorised the study (Approval No. QYFY WZLL 28569). Additionally, the Ethics Committee of the Affiliated Hospital of Qingdao University waived the requirement for written informed consent due to this study’s retrospective design.
Data collection
The clinical data of 90 patients with UC who met the inclusion and exclusion criteria was collected. The data included age at first VDZ dose, sex, body mass index (BMI), history of smoking, disease duration, disease extent and severity, baseline MES, comorbidities, extra-intestinal manifestations, perianal disease, bowel-related surgery (included appendicectomy and colectomy), and previous and concomitant therapies (5-aminosalicylic acid, steroids, immunosuppressants, and biologics). The laboratory findings included white blood cell (WBC) counts, haemoglobin (HGB) levels, platelet (PLT) counts, albumin (Alb) levels, C-reactive protein (CRP) levels, erythrocyte sedimentation rate (ESR), and neutrophil and lymphocyte counts. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The platelet-to-lymphocyte ratio (PLR) was calculated by dividing the absolute platelet count by the absolute lymphocyte count.
Follow-up clinical assessments were performed at weeks 6, 14, 22, 38, 46, and 54. At each visit, disease activity, endoscopic activity, laboratory findings, VDZ optimisation (between Q4W or Q6W), and adverse events were recorded. The data were manually extracted from the electronic medical records (EMR) system. The chart reviews were conducted by two trained medical researchers, who were carefully selected for their expertise and experience in handling medical data. To ensure the accuracy and consistency of the data extraction, each researcher was required to perform a self- review of the extracted data to identify and correct any potential errors. Discrepancies between the two reviewers were resolved through discussion and reference back to the original EMR.
Definition
According to the Montreal classification, UC was categorized into three forms: proctitis (E1), left-sided UC (E2), and extensive colitis (E3). The severity of the disease activity is delineated into three grades—mild (3–5 points), moderate (6–10 points), and severe (11–12 points). This stratification is determined by the total Mayo score, which includes stool frequency, rectal bleeding, MES, and physician’s global assessment. The partial Mayo score (PMS) is the partial version of the Mayo score without endoscopic subscore. Specifically, the MES was used to assess endoscopic severity [13].
Outcome assessment
Clinical outcomes of VDZ were assessed using the PMS at weeks 6, 14, 22, 38, 46, and 54. Clinical response was identified by either a reduction of no less than 2 points from the baseline in the PMS (or at least 30%) accompanied by an absolute rectal bleeding score of ≤ 1 or a reduction of at least 1 point from baseline. Clinical remission was determined as PMS ≤ 2, with no sub-score above 1.
Steroid-free clinical remission (SFCR) was defined as clinical remission with no combination of steroids. MES ≤ 1 was defined as endoscopic remission [14].
LOR to VDZ was defined as a clinical relapse necessitating a reduction in the intervals between VDZ infusions or the addition or switching to other alternative therapies (such as corticosteroids, tofacitinib, or other biological agents) among the those who had responded during the maintenance phase.
The primary endpoint was clinical remission at weeks 6, 14, 22, 38, 46, and 54. The secondary endpoints of this study included the rate of clinical response and SFCR at each time point, the endoscopic remission rates at weeks 22 ± 8 and 54 ± 16, and LOR up to the last follow-up when less than 54 weeks.
All adverse events that occurred during the study were recorded and analysed.
Statistical analyses
All data were analysed using SPSS 24.0 (IBM Corp., Armonk, NY, United States). Normally distributed data are presented as means ± standard deviation (SD), whereas non-normally distributed data are expressed as medians and interquartile range (IQR). Qualitative data are expressed as percentages (or rates). The comparable analysis of continuous variables was performed using the t-test or Mann–Whitney U test, while categorical variables were analysed using the chi-squared or Fisher’s exact test. A Kaplan–Meier analysis was conducted to estimate the cumulative rates of LOR at weeks 6, 14, 22, 38, 46, and 54. Univariate and multivariate Cox regression analysis was used to test the association between various factors and LOR in elderly and younger groups, selecting for risk factors from baseline patient characteristics. Schoenfeld residuals and log-log plots were used to assess the proportional-hazards assumption in Cox regression, and only variables without significant violations were included in the model. Multivariate logistic regression analysis was used to test the relationship between various factors and adverse events in the elderly group. Variables with a P value of < 0.05 in univariate analysis were further included in multivariable logistic regression analysis. The variance inflation factors (VIF) were calculated to evaluate multicollinearity, and a threshold of VIF < 5 was set as acceptable. Variables with a P value of < 0.05 in univariate analysis were further included in multivariable logistic regression analysis. Statistical significance was set at P < 0.05.