Individual morphological characters and their phylogenetic significance
Several morphological characters observed in Carbonoperlidae support the attribution to the stonefly total group (i.e., stem + crown groups). Below, we discuss individual characters and their significance for phylogenetic placement, first from the anterior to the posterior area of the forewing. Finally, we summarize the delimitation of the newly designated family from potentially related taxa.
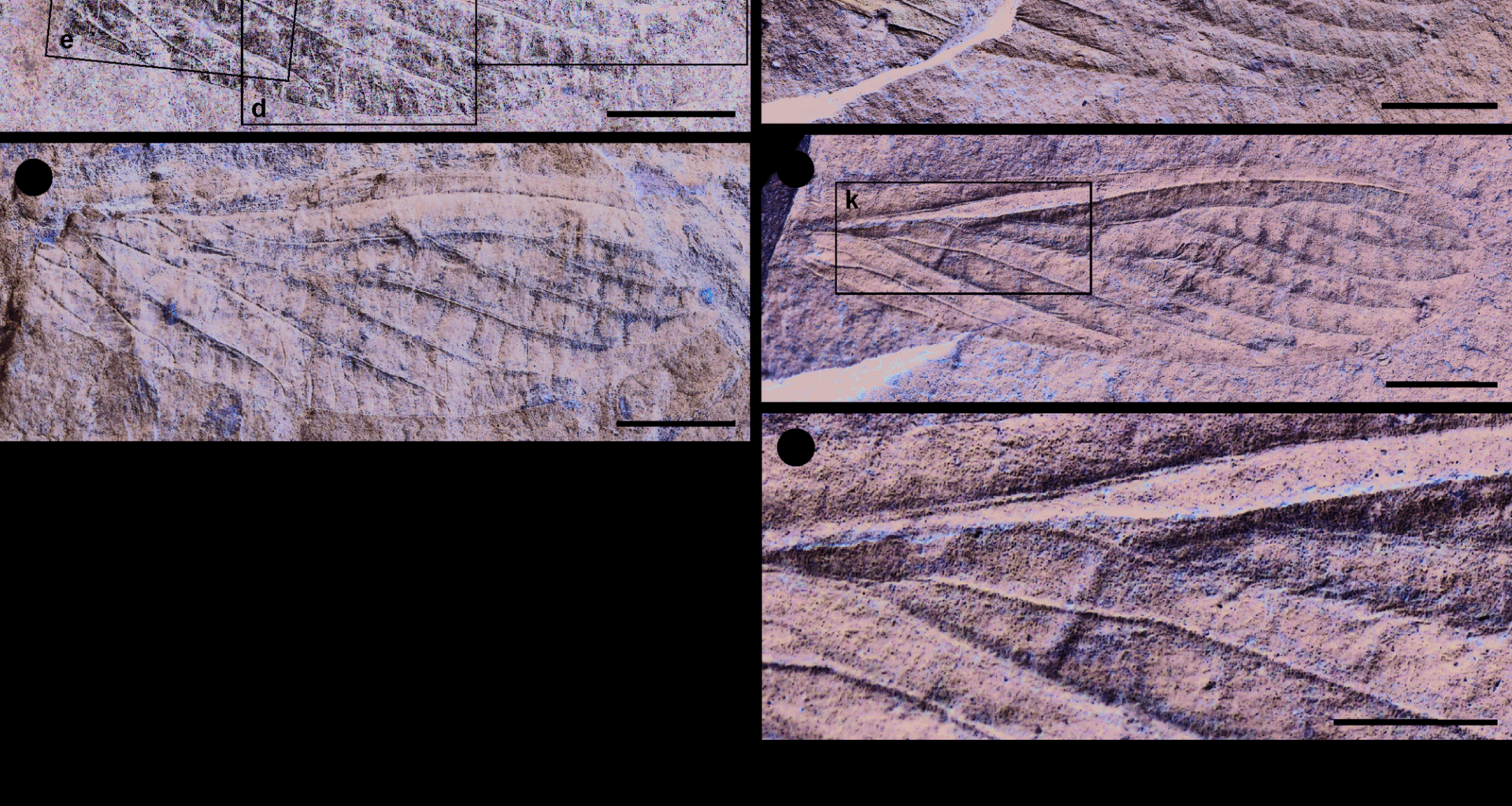
In all Piesberg fossils, ScP reaches RA (at about 1/2–3/5 of the wing length, Figs. 1a-c, f, h-j, l and m and 2a-c and h-j). According to Béthoux et al. [4], ScP reaching RA is a plecopteran feature, also present in stem-group representatives such as G. carpenteri. This trait is found in all extant stoneflies, except for some Antarctoperlaria, where ScP reaches the anterior wing margin. However, ScP reaching RA is not unique to stoneflies; it is also found in some taxa originally placed in Grylloblattida [16], such as the families Narkeminidae (figs 110-118 in Strozhenko[16]) and Tillyardembiidae (fig. 168 in Strozhenko[16]). Some affinity of the latter family to stoneflies has been suggested [21], although its inclusion in the stem group of stoneflies was questioned by Béthoux et al. [4]. Both families were later placed into the extinct order Cneminolestodea within stem-Orthoptera, where ScP reaching RA is also common [17, 18]. According to Schubnel et al. [6], whether ScP terminates on R or not is a variable character, even in extant insects (see e.g., Breitkreuz et al. [19]). Therefore, we consider that the presence of ScP reaching RA supports an affinity with Plecoptera but does not provide unambiguous evidence.
The course of the radial vein in the new material is congruent with that of Plecoptera in several respects. The RA/RP bifurcation is positioned relatively basally, at approximately 1/4–1/3 of the wing length (Figs. 1a-c, e and h-m and 2a-c). Such basal R bifurcation is considered a plesiomorphic condition in Plecoptera [4]. However, it also occurrs in other lineages, such as stem-Orthoptera or stem-Grylloblattodea [16, 20]. The radius posterior in Carbonoperlidae is only three- to four-branched (Figs. 1a-c, g-j and l-m and 2a-c and h-j), which aligns with the branching pattern seen in many extant stoneflies, where the number of RP branches is typically reduced. This is also the case of stonefly taxa known from the Permian [9, 22]. In the radial sector, Béthoux et al. [4] mentioned a characteristic forewing venation trait of stoneflies, namely the presence of a “specialized ra-rp cross-vein”. The same character was mentioned by Aristov [17] as the “sharp curves of the stem of RP at points where strong cross-veins ra-rp and rp-m join this stem”. Béthoux et al. [4] considered this cross-vein an apomorphy of all stoneflies, except for G. carpenteri. It has also not been documented in other stem-stoneflies from the family Gulouidae [6]. Carbonoperlidae similarly lacks this specialized ra-rp (and/or rp-m) cross-vein, and the RP forms no sharp curves (Figs. 1a, h and l and 2a and h). Instead, Carbonoperlidae fossils exhibit multiple, sub-identical cross-veins in the RA-RP area, none prominent, a pattern similar to that in Gulouidae. In Permian stoneflies, the specialized ra-rp cross-vein is universally present (see, among others, [9, 22]). Therefore, this character suggests that Carbonoperlidae holds a rather basal phylogenetic position relative to all stoneflies from the Permian onward.
In Carbonoperlidae, the stem of the Media is closely parallel to RP before sharply diverging from it (Figs. 1a, h and l and 2a), a pattern very similar to extant stoneflies. The Media in the new Piesberg material is two-branched, representing an ancestral condition in stoneflies [57]. Each of the M branches in Carbonoperlidae is simple, with all connecting veins representing only cross-veins. In contrast, stem-stoneflies from the family Gulouidae exhibit further distal branching of the medial veins [4, 6]. Such distal branching of MP is a rare trait even in extant stonefly species, where it has been interpreted as an atavism [4, 63]. The simple MP represents an apomorphy of Plecoptera, absent in Gulouidae, but already present in Permian stonefly taxa [9, 22] and the new material from Piesberg.
It should be noted that the homologization of the stonefly medial sector has been a matter of debate due to the unclear polarity of its veins (see brief discussion in Carpenter [9]; and references therein). Béthoux [57] recommended not distinguishing between MA and MP in stoneflies, opting to refer to all medial veins as M due to uncertain homology in this sector. Later, in Béthoux et al. [4], MA and MP are used for the M branches in G. carpenteri, although no statement about their polarity is provided, and this is probably unrecognizable in the fossils. In G. oudardi, Schubnel et al. [6] described the M stem as concave, dividing into two equally concave branches. This pattern corresponds with extant stoneflies, where both branches of M appear neutral but, upon dissection, are actually concave [57]. In Carbonoperlidae, the polarity of M branches is not distinguishable; we follow the nomenclature of Béthoux et al. [4] and Schubnel et al. [6] for the veins of the medial sector.
Another important character of the Media is the partial desclerotization of its stem or branches. The presence and precise localization of this desclerotization have been used to discuss the possible attribution of several lineages of Grylloblattida to stem-stoneflies [21]; and see Cui et al. [62]. In Carbonoperlidae, no desclerotization of the stem of the Media is visible. In the holotype of Carbonoperla sowiaki gen. et sp. nov., the course of Media is indistinct at the point of its branching (Fig. 1a). However, the structure of the matrix in that area appears altered, as shown in the ESEM photograph (Fig. 1d). Therefore, the weak visibility of the Media is likely an artifact of preservation rather than evidence of desclerotization.
The arrangement of the arculus varies in Carbonoperlidae. In Carbonoperla sowiaki gen. et sp. nov., no distinct arculus is present in either the holotype or paratype, whereas it is clearly visible in the holotype of Carbonopteryx heisingi gen. et sp. nov. (Pal cl. 16, Fig. 2a, c, d). The arculus is defined as a strengthened cross-vein, located as a first sclerotized structure in the area between M and CuA, and appears more robust compared to other cross-veins in the same field [57]. In stoneflies, the arculus typically connects CuA, just distal to its origin, with M, basal to the divergence of M from R. It represents a robust character, in particular when present in both fore- and hind wing (this combination is unique to stoneflies, see Béthoux et al. [4]). While generally present in Plecoptera, in some cases, only identical cross-veins occur between M and CuA [57]. The presence of a single strengthened structure in this area likely provides a mechanical advantage, assuming from the convergent acquisition of similar structures in a similar location in other fossil taxa unrelated to Plecoptera [57]. Some Grylloblattida also possess an arculus, referred to as ‘M5’ in various accounts, including Strozhenko [26]. Therefore, the presence or absence of an arculus does not serve as a conclusive character for determining the relatedness of Carbonoperlidae to Plecoptera.
In Carbonoperlidae fossils, the Cubitus is divided into CuA and CuP near the base of the wing (Figs. 1a, e and h and 2a and d). CuA is either simple (in Carbonoperla sowiaki gen. et sp. nov. holotype and paratype) or shows a fork (in Carbonopteryx heisingi gen. et sp. nov. holotype). Béthoux et al. [4] considered simple CuA to be the ancestral condition in Plecoptera. The Carboniferous stem-stonefly genera Gulou and Avionoptera exhibit CuA with a few very distal branches [4, 6], and none show the long branches of CuA. This character distinguishes Gulouidae and Carbonoperlidae from Grylloblattida, where CuA is divided into two main stems near the wing base [26].
In Fatjanoptera mnemonica Martynova, 1961 [27] (Fatjanopteridae), which was considered a stem-stonefly by Schubnel et al. [6], the structure of Cubitus is markedly different from all other stoneflies, including their stem-group relatives. According to the data of Martynova [27]; fig. 2, both branches, CuA and CuP, do not follow a nearly straight course with clearly opposite polarity, but instead run in a zig-zag pattern, with the polarity of the CuP vein being indistinct. Additionally, there is a tendency to form irregular cells between CuA and MP. The weakly developed corrugation of the CuP vein is highly unusual within Polyneoptera, while it occurs in Holometabola. Based on these characters, we restore Fatjanoptera mnemonica as a representative of Eumetabola, with a probable assignment to Holometabola (see also Prokop et al. [28]).
The Postcubitus (PCu) is basally connected to the first anal vein (Fig. 1a, e, h-l). This pattern is well visible in all Carbonoperlidae specimens with the forewing base preserved and does not represent an artifact. It is reminiscent of the arrangement in some extant stoneflies, where PCu is connected to a more basal A1 by a prominent cross-vein, closing the so-called anal cell [29]. However, in Carbonoperlidae, the short basal vein sections are nearly parallel and form an acute angle at their point of fusion, which is not congruent with the anal cell of extant stoneflies, where the pcu-a1 cross-vein forms a much more obtuse angle with PCu. We consider it more likely that the pattern in Carbonoperlidae represents the fusion of PCu and the first anal vein A1. This arrangement is different from Gulouidae and most extant stoneflies, although a similar fusion occurs in the extant stonefly family Styloperlidae, as described by Liu et al. [30], where the respective veins are treated as A1 and A2. In Styloperlidae, the veins PCu and A1 again separate distally, which is not the case in Carbonoperlidae. Remaining anal veins are reduced in number in Carbonoperlidae, with a single A2 observable in all the species described herein. Similar reduction of the forewing anal field is observable in extant stoneflies, whereas anal veins are more numerous in G. carpenteri [4].
Most of the newly discovered Carbonoperlidae fossils consist of isolated forewings, and the body structures cannot be discussed in these specimens. Only in the holotype of Carbonopteryx heisingi gen. et sp. nov. (Pal cl 16a/b) some body parts are preserved together with the wings (Fig. 2b, c). The most significant is the apparent external ovipositor (Fig. 2e–g). Due to preservation, we cannot observe the basal parts of ovipositor valvulae, but distal parts show two slightly curved gonapophyses of segments VIII and IX with a prominent ball-and-socket locking mechanism, the so-called olistheter interlocking gp9 onto gp8. This primary olistheter consisted of aulax (groove on gp8) and rhachis (ridge on gp9) and occurs widely among insects like Archaeognatha, Zygentoma, Odonata, Orthoptera, Grylloblattodea, Hymenoptera and extinct Palaeodictyopterida [32, 36]. Representatives of Orthoptera, including extinct relatives, possess a variety of additional olistheters, including one interlocking gs9 onto gp9 [49]. However, such additional olistheter is not discernible in C. heisingi gen. et sp. nov.
In extant stoneflies, the primary ovipositor is always missing. However, its presence is a presumable autapomorphy of Insecta [7, 10, 23, 52] and its presence in the stonefly stem group is not excluded; the disappearance of the primary ovipositor in the extant stoneflies might thus represent an apomorphy of the crown group. However, the ovipositor has never been reported even in the stem-stoneflies. The only known stem-stonefly with a preserved body is G. carpenteri. No ovipositor is present in this species, although the preservation of the body did not allow its detailed investigation; the description and attribution rely almost exclusively on the wing venation [4].
Despite the lack of the primary ovipositor in extant stoneflies, secondary ovipositors (formed by modified sternites and paraprocts) were independently acquired in three families (Gripopterygidae, Notonemouridae, Leuctridae), being used to insert eggs into crevices [35]. The purpose of the ovipositor in C. heisingi gen. et sp. nov. might have been similar. Gonapophyses VIII in this species have a series of regularly arranged short oblique lateral ribs and other microstructures (Fig. 2g), which could allow smooth penetration into the substrate or crevices. For classical endophytic oviposition, we would expect to find comparable surface microstructures on the gonapophyses IX, like those found in dragonfly Epiophlebia superleses or some zygopterous dragonflies [31]. The cutting valves are adapted for penetrating the plant tissue, being pointed and equipped with tooth-like projections [37]. Such a structure does not resemble the ovipositor in C. heisingi gen. et sp. nov. Hence, we instead speculate that C. heisingi oviposited into crevices or soil substrate close to the water.
Summary on the phylogenetic attribution of Carbonoperlidae fam. nov
Among the stonefly taxa potentially related to Carbonoperlidae, all Permian families can be excluded due to the absence of a specialized ra-rp cross-vein and associated inflection point of RP at the point of its insertion in Carbonoperlidae. A specialized ra-rp cross-vein is present in both the Permian taxa and all extant stonefly families. Affinities with the stem-stonefly family Gulouidae can be excluded based on the presence of a distally branched MP. The MP is simple in Carbonoperlidae, and this character is cited by Béthoux et al. [4] as distinguishing Gulou from all known stonefly taxa (along with the absence of the specialized ra-rp cross-vein, a character shared with Carbonoperlidae). The family Fatjanopteridae, with the type genus Fatjanoptera (previously considered another stem-stonefly lineage), is removed from the Plecoptera total group and also Polyneoptera herein and transferred to Holometabola based on the structure of the cubital veins (see above) and the irregular pattern of cross-veins.
In some earlier literature, stem-stoneflies were partially confused with certain taxa of Archaeorthoptera (sensu Béthoux and Nel [20]). Specifically, Aristov [17] synonymized Gulouidae with Emphylopteridae, a family assigned within the order Cnemidolestida (sensu Aristov [17]). However, this synonymy was refuted by Schubnel et al. [6], who pointed out that Emphylopteridae belong to Archaeorthoptera based on the characteristic venation pattern described by Béthoux and Nel [20]: the presence of a common stem M + CuA and the fusion of the free part of CuA with a branch of CuP. Both Gulouidae and Carbonoperlidae clearly differ from this basal branching pattern of veins, and any relationship with Emphylopteridae or any other Archaeorthoptera lineage can be excluded. The same character also excludes affinities of Carbonoperlidae to the small archaeorthopteran order Caloneurodea, whose representatives exhibit several similarities to Carbonoperlidae (and stoneflies in general) in the pattern of terminal branching of R and M. Some species described as Caloneurodea based only on distal wing fragment, like Aviobiella garroustei Nel & Roques, 2021 might even be related to Carbonoperlidae; this issue is not possible to resolve unambiguously, since the basal portion of the wing in A. garroustei is missing [67]. However, there are clear differences between Aviobiella and Carbonoperlidae, as the costal area is broad on the level of RA/RP and apical end of ScP posteriad of M fork in Carbonoperla and Carbonopteryx, unlike in Aviobiella.
Grylloblattida (sensu Strozhenko [16]) are excluded as they have at least two long CuA branches and MP desclerotized near its origin [50, 62]. Carbonoperlidae have a simple CuA (or possibly with a very distal twig in C. heisingi gen. et sp. nov., Fig. 2a) and no desclerotization of MP. Some taxa included in Strozhenko’s Grylloblattida were considered to form separate lineages at the stem of Plecoptera. This includes, in particular, the families Spanioderidae and Tillyardembiidae, rendering Grylloblattida (sensu Strozhenko [16]) paraphyletic [21]. The possible relationship of these taxa to stoneflies was discussed in detail by Béthoux et al. [4]. The affinity of Spanioderidae was considered not grounded, whereas the position of Tillyardembiidae was evaluated as uncertain. Tillyardembiidae actually exhibit some similarities with the Piesberg material, in particular C. sowiaki, where the area between CuA and wing margin is filled with long and sigmoidal cross-veins, resembling the backward comb of CuA occurring in Tillyardembiidae. However, in Tillyrdembiidae, this comb is formed by regular branches of CuA, seven in number according to Strozhenko [16]. Tillyardembiidae are also characterized by a wide RA/RP area [21], contrary to stoneflies including Carbonoperlidae. Aristov [18] characterizes Tillyardembiidae also by abundant branching of RP (rather reduced in stoneflies, including Carbonoperlidae) and places the family in Cnemidolestida, well-distinguishable from stoneflies based on the archaeorthopteran pattern of M + CuA (see above).
We conclude that the Piesberg taxa described herein cannot be attributed to any taxon of a rank higher then the genus. Therefore, we establish a new family, Carbonoperlidae fam. nov., to accommodate the newly described taxa.
Definition of the stonefly stem group and the taxa included
According to the original concept of a stem group, as proposed by Hennig [7], the stem group of a clade consists of fossils that belong to the clade but cannot be shown to belong to the crown group (the group containing all extant species of the clade, their last common ancestor, and all of that ancestor’s descendants). Thus, the stem group includes species in the direct line of descent of extant species, as well as side branches of the phylogenetic tree. For stoneflies, the ordinal name ‘Plecoptera’ is often used to refer to the total group, i.e., all taxa composing the stem and crown group. For example, Béthoux et al. [4] and Schubnel et al. [6] both described fossil taxa, which they explicitly treated as representatives of the stonefly stem group. Yet, they assigned them to the order ‘Plecoptera’.
Various taxa have been treated as members of the stonefly stem group in the literature. The placement of families Spanioderidae and Tillyardembiidae within the stonefly stem group was suggested by Aristov and Rasnitsyn [21] but later questioned by Béthoux et al. [4]. Jouault et al. [2] restricted the stem-Plecoptera to the Carboniferous families Guloidae and Fatjanopteridae, although the placement of the latter family is refuted herein. However, various authors have also considered some Permian stoneflies as stem-Plecoptera. Béthoux et al. [4] claimed that the Permian species Palaeoperla exacta Sharov, 1961 and Perlopsis filicornis Martynov, 1940 represent “genuine Permian stem-Plecoptera”, though they did not explain why those taxa do not belong to the crown-group. Sinitshenkova [12] treated all Permian stonefly taxa as belonging to the crown group. Wolfe et al. [13] selected Palaeotaeniopteryx elegans Sharov, 1961 (family Palaeonemouridae) from the Permian of Russia as the calibration fossil for crown-Plecoptera, based on unspecified synapomorphies. In summary, there is no concesus regarding the phylogenetic affinity of many Palaeozoic (mostly Permian) stoneflies to extant clades. The problem lies in the lack of a clear definition of crown-group stonefly taxa based on apomorphic characters that are applicable to fossil material. As a result, the term ‘stem-stoneflies’ is sometimes erroneously used to simply refer to ‘ancient’ stoneflies.
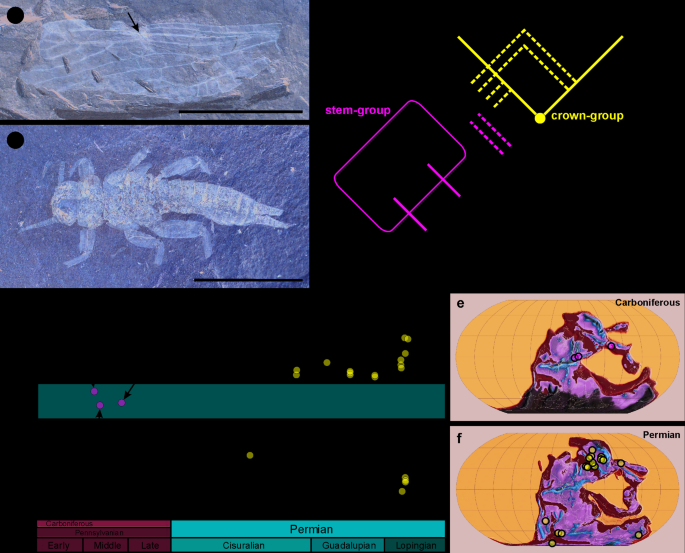
A potentially useful character for determining whether a taxon belongs to the stem or crown group is the presence of a single specialized ra-rp cross-vein and an abrupt angle of RP at its insertion in the forewing (Fig. 3a). This character is an apomorphy universally present in both extant suborders, Arctoperlaria and Antarctoperlaria, whereas more numerous, unspecialized cross-veins are present between RA and RP in Carboniferous stem-group taxa, including all species described herein (Figs. 1a, h and l and 2a and h). Although this character does not, by itself, define the crown group, no taxon exhibiting the specialized ra-rp cross-vein is known, whose placement in the crown group could be refuted based on other characters.
Nevertheless, the placement of the Permian taxa remains largely uncertain. We cannot confidently assign the Permian families to either of the stonefly suborders, as the diagnostic apomorphies for these suborders within the crown-group Plecoptera pertain to muscles or cellular structures [14], which are not observable in fossil specimens. However, all Permian taxa possess a specialized ra-rp cross-vein and no known character precludes their placement as early branches of Arctoperlaria or Antarctoperlaria (Fig. 3c). To distinguish between the genuine stem-group taxa from the remainder of stoneflies, we define the taxon Plecopterida to encompass the total-group of stoneflies. We also propose limiting the use of the ordinal name Plecoptera to those taxa that exhibit a specialized ra-rp cross-vein in the forewing, as found in all crown-group taxa. The age estimates of the crown group also vary. Grimaldi and Engel [10] presented a cladogram in which the Permian families Palaeonemouridae, Palaeoperlidae, and Perlopseidae were placed in the stem-Plecoptera, while Euxenoperlidae (containing Permian taxa from South Africa) was considered stem-Antarctoperlaria, i.e., within the crown-Plecoptera. Misof et al. [33] placed the age of the stonefly crown group at the Middle Jurassic (ca. 167 Ma), which is likely an underestimation since extant families were already reported from the Middle or Late Jurassic [40, 61]. Cui et al. [61] estimate the origin of the crown group to be between the late Carboniferous and Early Triassic. Letsch et al. [11] proposed that crown-group stoneflies evolved in the Permian (ca. 265 Ma) from a common ancestor inhabiting the northern part of Pangea before its break-up, whereas the stem group was considered to be distributed globally at that time. Therefore, Gondwanan plecopteran fossils from the Permian were automatically regarded as stem-group representatives by Letsch et al. [11]. Nevertheless, no morphological characters supporting the attribution to either the stem or crown group were mentioned. In fact, the Permian Gondwanan stoneflies (and Permian stoneflies in general) are very similar to extant forms, the original authors of these taxa sometimes even placed them within extant families [41, 42] and no convincing morphological character precludes their placement in the crown group. One possible scenario is that Permian Gondwanan stoneflies were members of the crown group that dispersed to the southern part of Pangea shortly after the crown group emerged, only to become extinct during the end-Permian mass extinction, which is known to have significantly affected stoneflies [2]. Several crown-stonefly lineages from the North later again dispersed to the emerging Gondwana, including Notonemouridae and Antarctoperlaria around 180 Ma [11, 40, 61], Acroneuriinae around 67 Ma [11], and Perlidae (genus Neoperla) in the Miocene or Pliocene [14].
Distinction between stonefly stem- and crown-group. a Forewing of Stenoperlidium permianum Tillyard, 1935, specimen In.46,393 (Permian of Australia, collection of the Natural History Museum, London, photo by J. Prokop), arrow marks specialized ra-rp cross-vein. b larva of Tshekardoperla depicta Sinitshenkova, 1987; specimen PIN 1700/1263 (Permian of Russia, collection of Borissiak Palaeontological Institute, Moscow, photo by J. Prokop). c Schematic cladogram depicting phylogenetic relationships at stem of Plecoptera and diagnostic apomorphies. d Spatiotemporal distribution of localities with stonefly fossils. Equatorial belt marked by pale red stripe. e Palaeogeographic position of Carboniferous localities. f Palaeogeographic position of Permian localities. Maps generated using GPlates 2.3.0 [43] and raster images from Scotese [44]. Scale bars are a, b = 10 mm
Origin and diversification of the stonefly crown group and the demise of the stem group
According to Hennig [7], the existence of the stem group provides direct evidence that the corresponding crown group was likely in the process of arising, having not yet acquired its complete set of characters. In stoneflies, all undisputed stem-group taxa occurred during the Carboniferous and this period possibly corresponds to when the crown group originated and further diversified during the Permian (although latest published time estimates infer younger age of the crown group [11]).
Budd and Mann [56] modelled the diversity of the stem- and crown-groups using a birth-death model. They found that as the crown group forms, the stem group quickly collapses, first into obscurity and then to extinction. The possibility that crown groups form ‘silently’ and remain as low diversity components of the background while the stem group diversifies is unlikely according to their model. This strengthens interpretations of the fossil record, viewing it as a relatively accurate reflection of diversification patterns, although notable exceptions are documented. For example, stem- and crown-group ants coexisted throughout the Cretaceous period [34].
For stoneflies in particular, this suggests that the crown group might have emerged as early as the late Carboniferous and came to dominate diversity during the Permian. By then, we already have many stonefly fossils with relatively modern wing venation, possibly representing the crown group, with undisputed stem-group representatives absent [15, 22]. With the sparse stonefly fossil record from the Carboniferous currently available, this scenario remains speculative. Nevertheless, it would align with the theoretical predictions of Budd and Mann [56], which propose that crown groups preferentially emerge after major mass extinctions.
Such a significant insect extinction event occurred during the Late Pennsylvanian, associated with a climatic transition from humid to arid conditions on equatorial Pangaea, along with the major extinction of dominant wetland communities [48, 58]. There was also a peak in insect origination during the early Permian (Artinskian stage), marking the appearance and diversification of derived Pennsylvanian clades that were smaller and suited to more xeric habitats [51].
Specifically, in stoneflies, the events at the Carboniferous-Permian boundary possibly reflect the demise of the stem group and the rise of the crown group. Although the Carboniferous-Permian transition itself represents one of the gaps in the plecopteran fossil record, it seems that the time between the Carboniferous and middle Permian was critical in stonefly evolution, marked by an increase in the origination rate around 300 Ma, as derived from theoretical models [2].
This period also coincided with a time of continental connexion, during which the distribution of Plecoptera would have been restricted due to arid areas in the centre of Pangea [11, 39]. When comparing the geographical distribution of fossil occurrences across the Carboniferous and Permian strata (Fig. 3d–f), it is evident that Carboniferous stem-group representatives were confined exclusively to the narrow tropical equatorial belt. In contrast, the Permian taxa (possibly representing crown group) were absent near the palaeoequator and instead occurred in higher palaeolatitudes on both hemispheres. This pattern does probably not represent a simple sampling bias, since multiple localities yielding fossil insects are distributed in other paleolatitudes during a given period (see Eskov [68], figs. 497 and 498). Obviously, the density of fossil insect occurrences is not distributed evenly throughout the globe (e.g., all Pennsylvanian konzentrat Lagerstätten that contain at least 100 described occurrences are tropical [51]). Therefore, any pattern shown by these data should be viewed with caution. Nevertheless, based on available data, we hypothesize that the crown-group stoneflies were better adapted to colder environmental conditions in temperate regions, associated with preferred larval development in cooler, well-oxygenated waters. Such habitats currently host the majority of stonefly diversity [1], as reflected in their prevalent distribution in higher latitudes– a pattern that possibly originated in the Permian.