Study design and participants
After local ethical approval, a convenience sample of 119 pwMS, 65 women and 54 men, aged 40.4 (SD = 11.4) from the Multiple Sclerosis Center, Sheba Medical Center, Tel-Hashomer, Israel, were admitted to the study. Participants were recruited through direct contact with the study’s staff or local advertising. While recruitment was based on a convenience sample, the Sheba MS Center is the largest in Israel and serves approximately 60% of the national MS population, with referrals from across the country. Nevertheless, the potential for selection bias remains. Inclusion criteria included: (1) a diagnosis of MS according to the revised McDonald Criteria 201716, and (2) an age range of 21–65 years. Exclusion criteria included: (1) major depression or cognitive decline limiting the ability to understand the study instructions or complete the study protocol, (2) pregnancy, (3) blurred vision, (4) MS clinical relapse or treatment with corticosteroid therapy within six months prior to the examination (5) and cardiovascular disorders. These criteria aimed to minimize confounding from non-MS-related factors. The study was approved by the Sheba Institutional Review Board (Ref# SMC-8328-21). All subjects signed an informed consent form before participation. All methods were carried out in accordance with relevant guidelines and regulations.
Study protocol
An experienced health professional performed and collected all outcome measures during a single session. The session included recording the participant’s demographic and clinical information (age, gender, disease duration, type of MS, family status, and home location). Subsequently, participants completed a questionnaire about their employment status over the past six months, including their average weekly working hours. Based on this, employment was categorized as full-time (30–40 h/week), part-time (less than 30 h/week), or unemployed (not currently working). Employed participants were also asked whether they had missed work due to MS during that period (yes/no), and if so, to report the total number of workdays missed. Furthermore, participants completed a self-report questionnaire on work difficulties (MS Work Difficulties Questionnaire). Next, they completed a battery of patient-reported outcome measures related to mobility, cognition, perceived fatigue, fear of falling, bladder and bowel function, depression, and anxiety. In addition, they completed testing of their information processing speed as an indicator of cognitive status. The Expanded Disability Status Scale (EDSS) score17 was retrieved from the patient’s computerized medical records.
Outcome measuresMS work difficulties questionnaire shortened scale
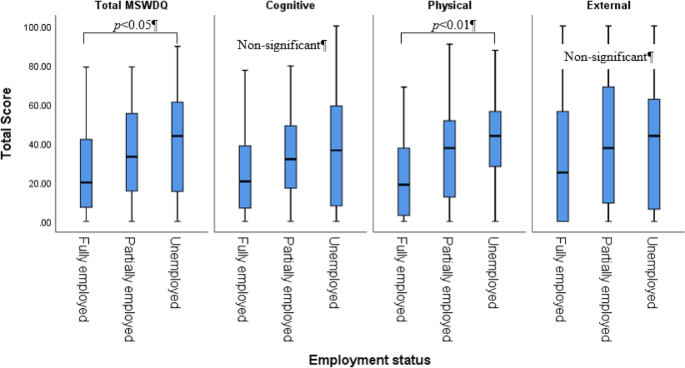
The MS Work Difficulties Questionnaire shortened scale (MSWDQ-23)18, a shortened self-report survey of the original MS Work Difficulties Questionnaire19 examined work difficulties in pwMS across three broad domains: psychological/cognitive, physical, and external barriers. Subscale scores for physical, psychological/cognitive, and external barriers are computed as a percentage by summing the observed item scores, divided by the total possible item scores in each subscale, and then multiplying the value by 100, presenting a maximum total score in each subscale of 100. The high scores indicated more significant difficulties. This survey has shown good concurrent validity to future work expectations in currently employed pwMS. A 2012 study found that Cronbach’s alpha values for the three subscales were 0.89, 0.81, and 0.80, respectively, indicating excellent internal consistency19.
Perceived fatigue
Perceived fatigue was assessed by the Modified Fatigue Impact Scale (MFIS), a multidimensional 21-item questionnaire that acquired data relating to the effects of fatigue-physical (9-items), psychosocial (2-items), and cognitive (10-items) domains over four weeks. pwMS rated the 21 items using a 5-point Likert-type scale, ranging from never (0) to always (4); the higher the score, the more perceived fatigue. The MFIS has demonstrated good reliability over a six-month period and has strongly correlated with the Fatigue Severity Scale results20.
Perceived mobility
Perceived mobility was measured by the 12-item MS Walking scale (MSWS-12). The MSWS-12, a valid self-reported questionnaire assessing walking ability in pwMS, is the most widely used patient-reported measure of perceived limitation in walking attributable to MS21. It has been used in numerous clinical trials in the pwMS population due to its excellent psychometric properties22,23.
Anxiety and depression
Anxiety and depression were assessed by the Hospital Anxiety and Depression Scale (HADS), widely used in clinical practice and validated in the pwMS population24. The scale, consisting of 7 items, rates two components – anxiety and depression. The questionnaire includes three cut-off scores indicative of different levels describing clinically relevant distress: a score between 0 and 7 = normal status, 8–10 = borderline, and 11–21 = an abnormal depression rate25.
Cognition
The Symbol Digit Modalities Test (SDMT), a cognitive outcome measurement tool was administered utilizing standardized procedures26. The SDMT is a measure of information processing speed. The primary SDMT outcome is the raw score. Substantial evidence supports the psychometric properties of the SDMT27,28 which has been recommended as the cognitive test of choice for MS clinical trials26.
Fear of falling
Fear of falling was assessed by the Falls Efficacy Scale International (FES-I) self-reported questionnaire29,30. The questionnaire assessed the level of concern regarding falling during 16 activities of daily living, ranging from basic to more demanding activities, including social activities that may have contributed to the participants’ quality of life. The level of concern for each item was scored using a four-point scale (1 = not at all concerned, 4 = very concerned). The total FES-I score ranges from 16 to 64, with higher scores indicating greater concern about falling. Although no universal cut-off is established for the MS population, scores above 23 typically suggest elevated fall-related concern. The FES-I has been shown as appropriate for research and clinical purposes in pwMS31.
Bladder and bowel function
Bladder and bowel function were assessed by the Bladder Control Scale (BLCS) and the Bowel Control Scale (BWCS). Both scales are components of the MS Quality of Life Inventory (MSQLI), which is both generic and MS-specific32. The BLCS (four-item instrument) and the BWCS (five-item instrument) provide a brief assessment of bladder/bowel control and how much bladder/bowel problems impact everyday activities. Higher scores indicate more problems. The BLCS and BWCS have a Cronbach’s alpha of 0.82 and 0.78, respectively.
Statistical analysis
Descriptive statistics were used to determine demographic characteristics (gender, home location, family status, and education), clinical characteristics (disease duration, disability (EDSS), type of MS), fear of falling, MS-related measures (mobility, bladder/bowel function, perceived fatigue, depression, anxiety, and cognition), and work-related measures (employment status and work difficulties via the MSWDQ-23). Only the total MFIS score was used in analyses to avoid multicollinearity with its subscales. Pearson’s correlation analysis examined the relationships between the outcome measures. A one-way ANOVA assessed differences in the outcome measures based on employment status (full-time/part-time/unemployed). Given the exploratory nature of the study, multiple ANOVAs and t-tests were performed without formal correction for multiple comparisons. While post hoc Tukey HSD tests were applied for pairwise group comparisons in the ANOVA, we acknowledge the increased risk of Type I error and advise cautious interpretation of the p-values.
We assessed normality of distributions using the Shapiro-Wilk test and examined homogeneity of variances with Levene’s test. Although certain variables (e.g., EDSS and fatigue scores) showed mild deviations from normality, ANOVA was retained given its robustness in moderately sized samples. We acknowledge the ordinal nature of the EDSS and note this as a limitation in applying parametric tests to this variable.
An ordinal logistic regression analysis was conducted to identify which study variables predicted employment status. The outcome variable was coded such that a higher value indicated a greater employment impairment (full-time = 1, part-time = 2, unemployed = 3). Only variables that showed significant differences between employment groups in the preceding one-way ANOVA analyses were included in the model. All selected variables were entered simultaneously into the regression model (i.e., no stepwise selection was applied). The proportional odds assumption was tested using the Test of Parallel Lines in SPSS and was not violated (p > 0.05), supporting the appropriateness of the ordinal logistic regression model. To assess multicollinearity among predictors, we calculated variance inflation factors (VIFs) for all included variables. All VIFs were below 2.0, indicating no significant multicollinearity.
Independent-sample t-tests examined the differences in outcome measures between employed (full or partly) pwMS who were or were not absent from work during the past 6 months. Unemployed patients were excluded from this analysis. Based on the t-test results, a logistic regression was conducted to determine which outcome measures best-predicted work absenteeism due to MS.
All analyses were performed using the SPSS software (version 28.0 for Windows; SPSS Inc., Chicago, IL, USA). The data were examined for normality violations, outliers, errors, and missing values. All reported p-values were two-tailed, with the significance level set at p < 0.05.