Data source
Data used in this study were obtained from GBD 2021, a comprehensive global research initiative that estimated key health metrics and assessed the impact of various factors on health across global, regional, and national levels. Specifically, GBD 2021 provided updated estimates of incidence, prevalence, mortality, years of life lost (YLLs), YLDs, and disability-adjusted life-years (DALYs) for 371 diseases and injuries, as well as 88 risk factors, spanning 204 countries and 811 subnational locations from 1990 to 2021 [13]. These estimates enabled synchronized resource allocation and facilitated cooperative initiatives to address interrelated health challenges.
For severe periodontitis prevalence estimation, GBD 2021 employed DisMod-MR 2.1, a Bayesian meta-regression tool, with stratification by age, sex, year, and country [13,14,15]. Mortality attributed to severe periodontitis was fixed at zero, as this condition is not typically fatal [13, 15]. Disability weight (DW), quantifying health impairment severity, were used to calculate YLDs through multiplication of total cases, duration until recovery or death, and DW [13]. The DW for severe periodontitis in GBD 2021 was 0.007 (95% uncertainty interval: 0.003–0.014). The modeling framework incorporated data from an updated systematic review of observational studies on severe periodontitis epidemiology and health survey [13, 16].
In this study, we retrieved the incidence and YLD data for severe periodontitis across all ages, 15 age-specific groups (15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85+), and age-standardized groups in BRICS-Plus countries from 1990 to 2021 for trend analysis.
Case definition and study population
In GBD 2021, disability associated with severe periodontitis was defined as “bad breath, a bad taste in the mouth, and gums that bleed a little from time to time, but which does not interfere with daily activities”, corresponding to ICD-10 codes K05.0–K05.6 and ICD-9 codes 523.0–523.9 [12, 17]. The case definition utilized a hierarchical approach prioritizing: Community Periodontal Index of Treatment Needs (CPITN) = 4, Clinical Attachment Loss (CAL) > 6 mm, and Gingival Pocket Depth (PD) > 5 mm [15]. These parameters were considered equivalently as reference definitions without additional cross-walking [12]. Data using alternative thresholds, (e.g., CPITN = 3, CAL > 5 mm, CAL > 4 mm) were cross-walked to the reference definitions using the Meta-regression tool (MR-BRT) to maximize data inclusion [12, 18]. Prevalence estimates were adjusted for edentulism to correct systematic overestimation bias arising from excluding edentulous populations in modeling [12].
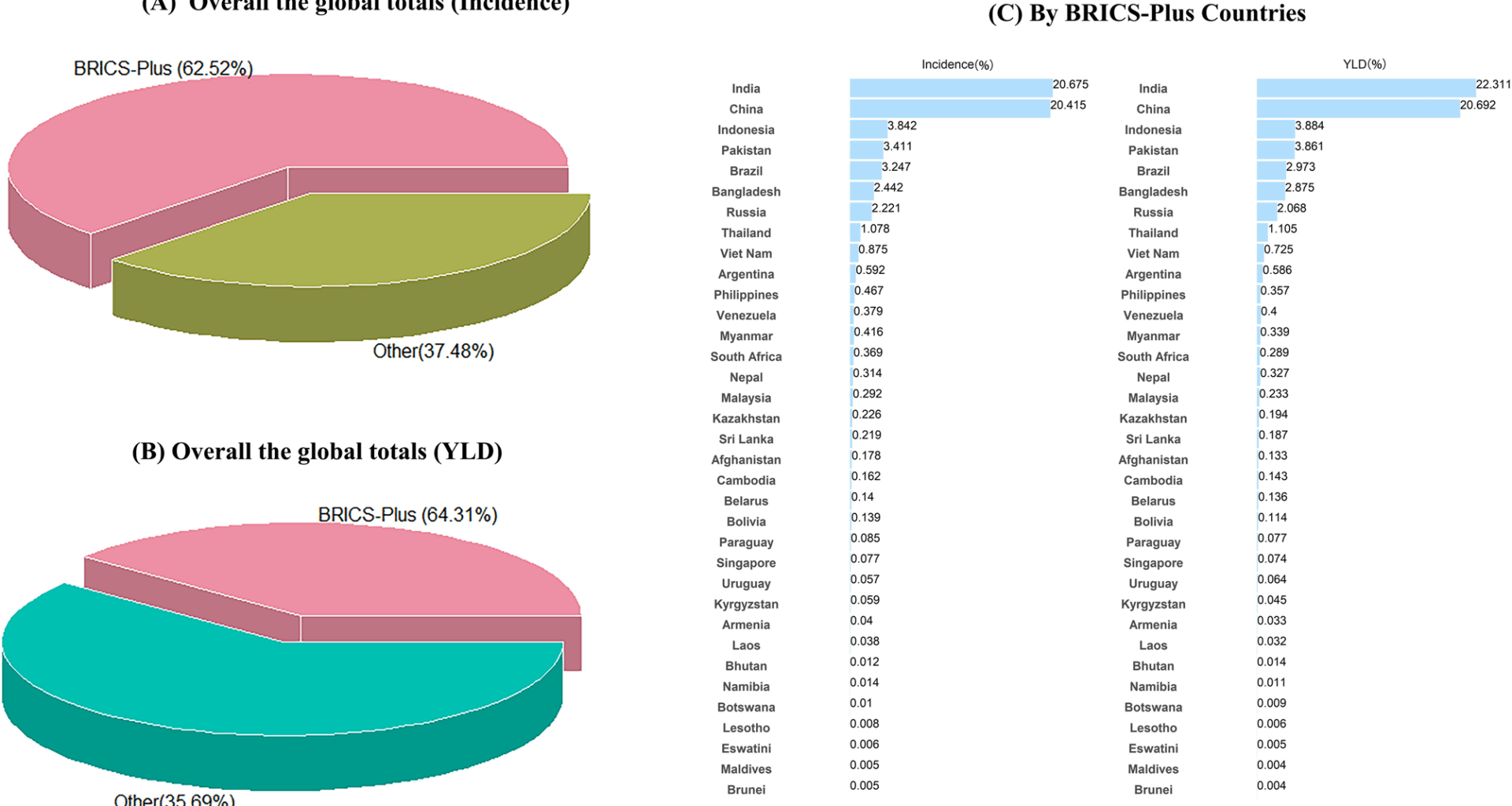
Our study population were restricted to BRICS-plus nations, categorized into five different regions: Mercosur, SACU, SAARC, China-ASEAN FTA and EEU and an independent subdivision for 35 nations [1]. These subdivisions included Mercosur (Brazil, Argentina, Paraguay, Uruguay, Bolivia, Venezuela), SAARC (Afghanistan, Bangladesh, Bhutan, India, the Maldives, Nepal, Pakistan, Sri Lanka), China-ASEAN FTA (China, Indonesia, Malaysia, Philippines, Singapore, Thailand, Brunei, Vietnam, Laos, Myanmar, Cambodia), SACU (Botswana, Lesotho, Namibia, South Africa, Swaziland), and EEU (Kyrgyzstan, Armenia, Belarus, Kazakhstan, Russia) [3].
Statistical analysis
In our study, we presented all estimates as counts, age-specific rates and age-standardized rates (ASR) per 100,000 population, and the ASR was calculated using the direct method with age-specific weights from the GBD standard population [19]. Specifically, the ASR per 100,000 was computed using the following formula: \({\rm{ASR}} = {{\mathop \sum \nolimits_{i = 1}^A {a_i}{w_i}} \over {\mathop \sum \nolimits_{i = 1}^A {w_i}}} \times 100,000 \) (ai was the ith age group and the population number (or weight wi) in the same age group i of the reference standard population [20]. The EAPC and corresponding 95% confidence intervals (CI) were computed to assess temporal trends in severe periodontitis burden using a linear regression model. In this model, the natural logarithm of the ASR (Y) was expressed as α+βX+ε, where X represented the calendar year and ε denoted the error term. The EAPC was then calculated as 100×[exp(β) − 1] [20]. A positive EAPC indicated increasing incidence or YLD rates over the 31-year period, while a negative value implied a decreasing trend. Additionally, the BAPC was employed to project severe periodontitis burden from 2022 to 2040, as this approach has shown superior performance and more reasonable predictions compared to other models [21]. The BAPC model, based on the age-period-cohort analysis, utilized a second-order random walk to smooth prior rates relating age, period and cohort effect, with the integrated nested Laplace approximations (INLA) package estimating marginal posterior distribution [22]. A major advantage of the BAPC model was its ability to address convergence issues commonly observed in traditional Bayesian approaches using Markov chain Monte Carlo sampling, thereby improving prediction reliability [22]. The BAPC and INLA packages within the R 4.3.2 software were used to develop a BAPC model, and p < 0.05 was regarded statistically significant.