The Food and Drug Administration (FDA) has designated Stoboclo® (denosumab-bmwo) and Osenvelt® (denosumab-bmwo) as interchangeable biosimilars to the reference products Prolia® (denosumab) and Xgeva® (denosumab), respectively.
Stoboclo is approved to treat postmenopausal women with osteoporosis at high risk for fracture, to increase bone mass in men with osteoporosis at high risk for fracture, to treat glucocorticoid-induced osteoporosis in men and women at high risk for fracture, to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer, and to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
Osenvelt is indicated to prevent skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors, to treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity, and to treat hypercalcemia of malignancy refractory to biphosphonate therapy.
The FDA approved Stoboclo and Osenvelt as biosimilars in March 2025. The interchangeability designation was based on the same clinical data package used for approval, which included a phase 3 trial (ClinicalTrials.gov Identifier: NCT04757376) that compared denosumab-bmwo to the reference product (Prolia) in postmenopausal women with osteoporosis.
Findings showed no clinically meaningful differences in efficacy, safety, pharmacokinetic, and immunogenicity data between denosumab-bmwo and Prolia at week 52. Comparable efficacy results were also observed at week 78 after switching to the biosimilar from the reference product.
The interchangeability designation allows Stoboclo and Osenvelt to be substituted at the pharmacy for the reference product without a prescriber’s consultation, subject to state pharmacy laws.
“Today’s [interchangeability] designations reinforce confidence in Stoboclo and Osenvelt among physicians and pharmacists, facilitating a more seamless switch from the reference products to our denosumab biosimilars,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA.
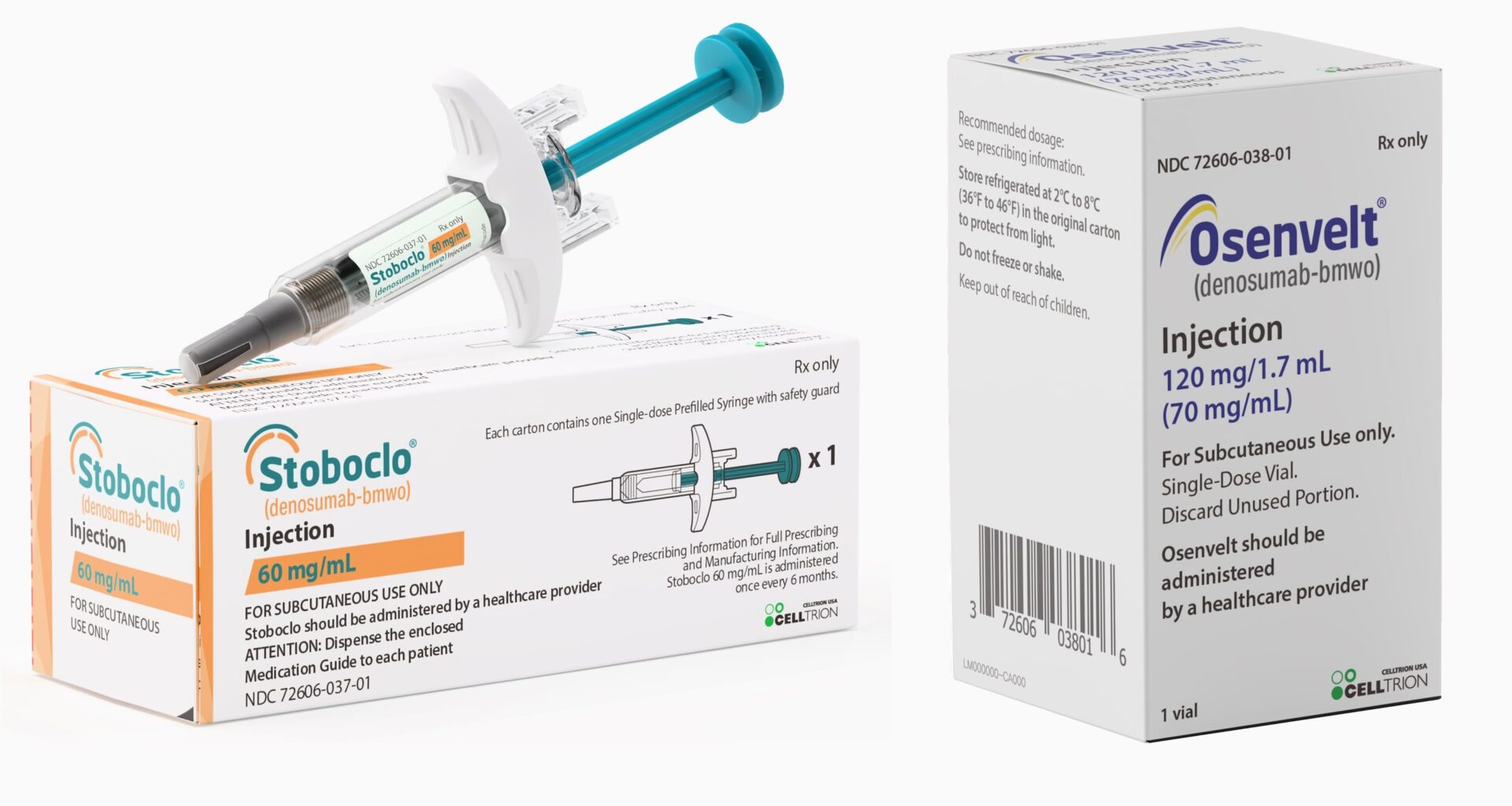
Stoboclo is supplied as a 60mg/mL solution in a single-dose prefilled syringe. Osenvelt is supplied as a 120mg/1.7mL solution in a single-dose vial.
This article originally appeared on MPR