Sometimes it’s necessary to break a few diamonds to figure out what’s happening on an alien planet’s surface. Or at least diamonds in diamond anvil cells, devices designed to squeeze materials to incredible pressures. Using these cells, planetary scientist Dan Shim and his team at Arizona State University have shown experimentally that reactions between magma and hydrogen produce a surprising amount of water. The results indicate that these reactions could be hydrating the surface of distant exoplanets (Nature 2025, DOI: 10.1038/s41586-025-09630-7).
The number of confirmed exoplanets has exploded in the past decade. Today, NASA has an inventory of over 6,000 planets beyond our solar system, each classified by their size and density. The range is vast—from gas giants larger than Jupiter to rocky terrestrial planets smaller than Earth. The most abundant type are planets sitting somewhere between Earth and Neptune size, called sub-Neptunes. There is nothing comparable in our solar system.
“We don’t exactly know the real nature of sub-Neptunes, but we can model their composition by knowing their density,” Shim says. Astronomers have done this for years. Using gravitational observations, they can calculate a planet’s density and then use that value to extrapolate its composition based on the most common elements in the universe.
Two general types of sub-Neptunes have emerged from these models: dry, hydrogen-rich worlds and wet, water-rich worlds, Shim says. The dry worlds can “have a thick atmosphere of hydrogen, and the interior is essentially magma,” he says. Theoretically, the gas should react with the molten rock. As someone with a background in chemistry, Shim wanted to know how that reaction would actually proceed.
The difficulty with these types of experiments, Shim says, is getting reaction materials, specifically hydrogen gas, to a temperature and pressure similar to those on the surface of a sub-Neptune exoplanet. During the team’s initial attempts to compress hydrogen gas, crushed olivine—a silicate mineral—and iron between diamond anvils, “we realized that hydrogen can diffuse into diamond anvils, and then it breaks the anvil material,” Shim says.
After a few years of development (and a few broken diamonds), the group successfully combined the diamond anvils with a high-powered pulsed laser to achieve pressures of up to 40 GPa and temperatures of above 3,000 K. Under these conditions, the hydrogen and silicates melt together. The researchers used X-ray diffraction and Raman spectroscopy to characterize the materials in the resulting reaction product. From these data, they were able to determine the specific redox reactions occurring between the olivine, iron, and hydrogen. Finally, using mass balance calculations, they quantified the amount of liquid water produced.
A gray-scale scanning electron microscope image that has a light-colored blob of material within a cavity of small crystals.
Using scanning electron microscopy, researchers in Dan Shim’s group identified iron-rich alloys (center, white) that formed from the reaction between hydrogen and silicate. Water also formed through this reaction.
Credit:
Harrison Horn/Arizona State University
The results were a surprise. “The theoretical prediction that was made before our study underestimates water content by a factor of 2000–5000,” Shim says. “What the experiment shows is that, in fact, the reaction produces enough water for a water world.”
Shim is not the only scientist probing the geochemistry of exoplanets with high-temperature, high-pressure laboratory experiments. At Carnegie Institution for Science, planetary geochemist Anat Shahar and her group also used diamond anvils and a laser to probe the chemistry of molten silicate glass and hydrogen gas at high temperatures and pressures. They also found evidence for water formation (Nature 2025, DOI: 10.1038/s41586-025-09816-z). Both researchers were surprised to see each other’s work published in Nature the same week; the research was done independently.
Unlike Shim’s study, “we start with no iron metal in our experiment,” Shahar says. Instead, her team uses an iron-containing silicate glass called pyrolite. The synthetic material is an analog of Earth’s primitive mantle. In an exoplanetary context, Shahar says, it’s similar to what a magma ocean would likely be composed of after the planet’s metallic core has formed.
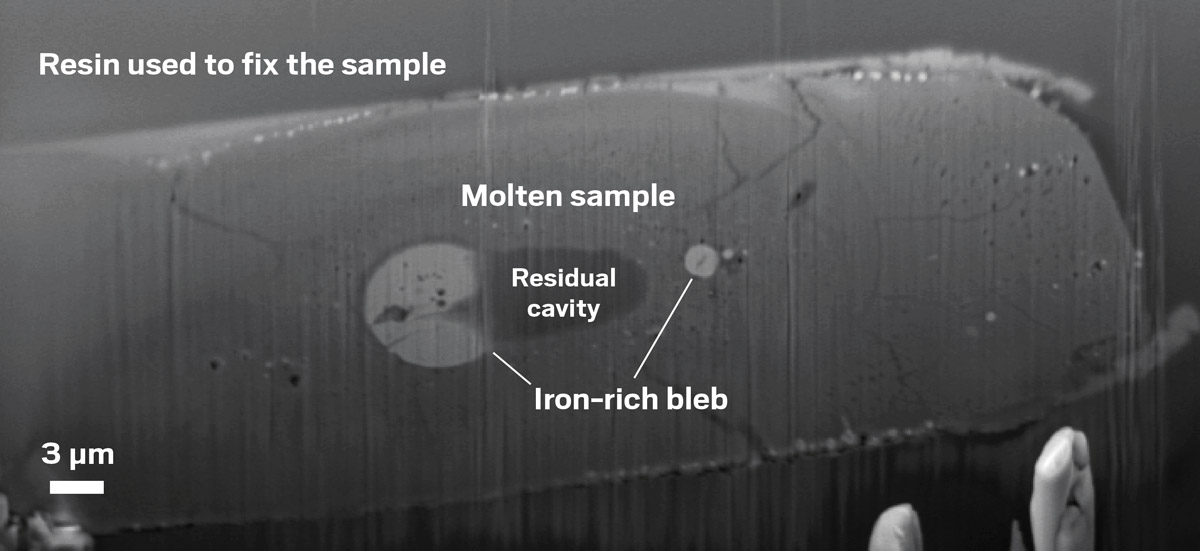
Upon reaching the extraordinary temperatures and pressures characteristic of sub-Neptunes, Shahar’s team, led by researcher Francesca Miozzi, saw indirect evidence for the formation of liquid water during the experiment: iron-rich blobs of material and a large, empty cavity that likely used to contain water. “The liquid water in our experiment comes from the reduction of the iron [oxide] that’s in the silicates to then form iron metal,” Shahar says.
A gray-scale scanning electron microscope image showing a sample that has lighter and darker regions. The light regions are labeled as iron-rich blebs, and the darker region is labeled as a residual cavity.
When researchers in Anat Shahar’s group combined molten silicate glass with hydrogen gas under high temperatures and high pressures and observed the resulting material using scanning electron microscopy, they found iron-rich blobs and empty cavities. The scientists attribute these products to the reduction of iron oxide to iron metal, which would produce the liquid water that likely filled the cavity during the experiment.
Credit:
Francesca Miozzi/Carnegie Institution for Science
Shahar’s team also used mass spectrometry to quantify the amount of hydrogen bound up in the final material. “We can’t know exactly how the hydrogen was bonded; we don’t know if it was H2, we don’t know if it was OH” because of limitations of the mass spectrometry technique, she says. But the results do show how the solubility of gaseous hydrogen into molten rock changes with temperature and pressure. “We found that it was mostly a function of temperature” rather than pressure, Shahar adds.
Shahar thinks the two papers complement each other well. “What’s cool is we come to basically the same conclusion,” she says. “They have direct evidence for water. We have direct evidence for hydrogen solubility, and we have indirect evidence for water,” Shahar adds. “It all makes sense together.”
The studies both suggest that a lot of water could be created through the geochemistry occurring on the surface of hot exoplanets with thick atmospheres, says planetary scientist Quentin Williams of the University of California, Santa Cruz. He was not involved in either study but authored the Nature commentary published alongside Shim’s work.
“That’s a big deal in terms of not only the water content but also what you’re doing to the hydrogen envelope and the magma ocean as well,” Williams says. Essentially, the results open a new avenue for understanding planetary evolution.
Scientists have assumed that wet planets collected water from their surroundings as they grew. In our own solar system, the most water-rich worlds—Uranus, Neptune, and the icy moons around Jupiter and Saturn—exist beyond the snow line, where temperatures drop low enough for gaseous water to freeze into ice grains and accrete onto budding planets.
But more observations revealed that many water-rich sub-Neptunes orbit their stars well within the snow line. Based on traditional models, such planets would need to grow in an area beyond the snow line and then migrate into their current orbits. But if an exoplanet can internally generate water, “you don’t have to move [it] in from someplace where ice condensed in the solar system,” Williams says. In effect, a dry world can become wet all on its own.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society