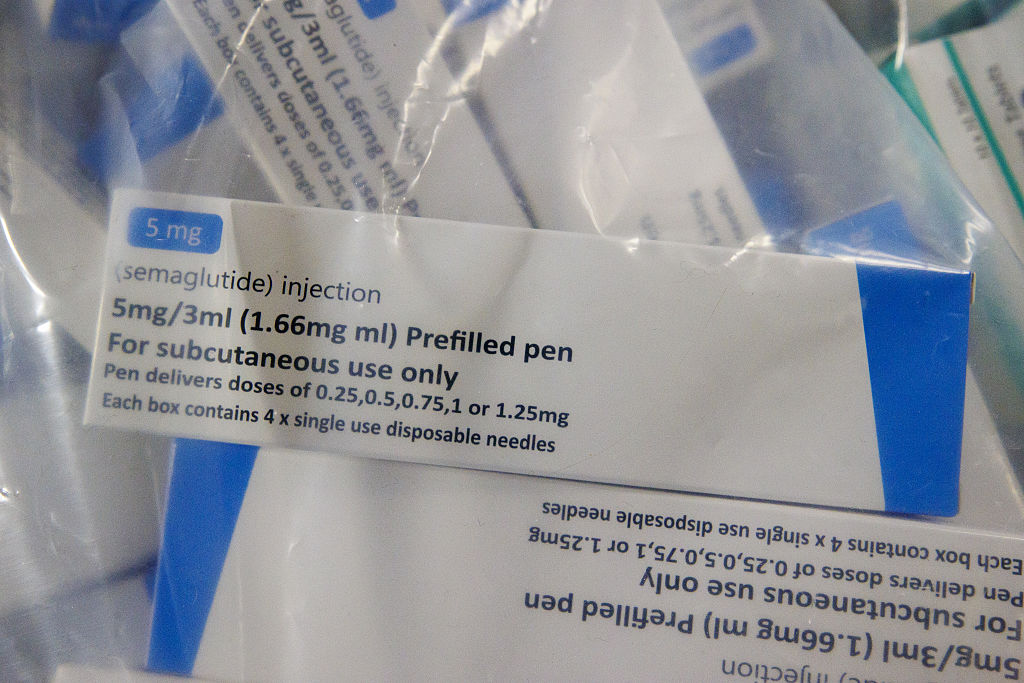
Danish drugmaker Novo Nordisk has warned that new EU rules could allow some pharmacies to compound their own, unauthorised versions of weight-loss medications – but a major pharmacist group insists it’s a false alarm.
According to a Novo spokesperson, an important loophole has emerged in the EU’s sweeping overhaul of its pharmaceutical legislation: certain pharmacies could be permitted to mix specific medicines, including versions of the company’s blockbuster weight-loss drugs Ozempic and Wegovy.
“Opening the doors to large-scale unregulated medicines that bypass the strict standards designed to keep patients safe would create a Wild West,” the spokesperson told Euractiv.
They added that the revisions “would allow pharmacists to stockpile, filling their shelves with product in anticipation of prescriptions rather than waiting for individual prescriptions.”
Currently, pharmacies may prepare compounded doses of medicines only in limited cases, such as during shortages or when lower-dose options aren’t available for children.
False alarm on fake medication?
But Ilaria Passarini, secretary general of the Pharmaceutical Group of the European Union (PGEU), which represents more than 400,000 community pharmacists, said Novo’s warning that pharmacies would be able to manufacture drugs more broadly as ‘parallel production’ is a false alarm.
“It’s pure madness,” she said. “The claims circulating that the revision would enable pharmacies to mass-produce or stockpile unregulated medicines, including complex injectables like GLP-1 analogues, are simply wrong.”
Community pharmacies, in particular, are “not asking to change or expand EU rules on compounding,” she said.
Industry has raised the alarm on a potential change in law that could allow large-scale pharmacies to prepare a few weeks’ worth of customised medicines in advance.
Third-party gateway
Novo Nordisk also fears that the new legislation could boost an already large, online third-party marketplace. In September, the European Medicines Agency (EMA) warned of a “sharp rise” in counterfeit medicines marketed and sold as GLP-1 agonists.
“If compounding restrictions in the EU are relaxed, it is highly likely that this unlicensed and unregulated supply chain will start to find its way to patients through legitimate pharmacies,” the Novo spokesperson explained.
Passarini countered that the current debate is about preserving “small-scale, need-driven” compounding in community settings, not “opening the door to copycat versions of innovative biologics.”
EU countries and lawmakers are still negotiating the pharma package, with a deal expected in December. Euractiv understands that the loophole could have been closed in more recent talks.
(bms, aw)