Major changes that will increase access to GLP-1 weight loss drugs—widely regarded as critical for weight management and metabolic health—for people who receive health care through Medicare or Medicaid have been announced by The Centers for Medicare & Medicaid Services (CMS).
GLP-1 medicines include semaglutide (familliar brand names: Wegovy, Ozempic and Rybelsus), tirzepatide (Mounjaro) and liraglutide (various brand names).
Why it Matters
The National Center for Health Statistics reports that more than 70 percent of U.S. adults aged 20 and above are classified as either overweight or obese. Obesity can lead to chronic, debilitating, and even life-threatening health issues such as diabetes, heart disease, and high blood pressure, so increased access to GLP-1 weight loss drugs could improve the long-term health, well-being, and outcome for millions of Americans.
 What To Know
What To Know
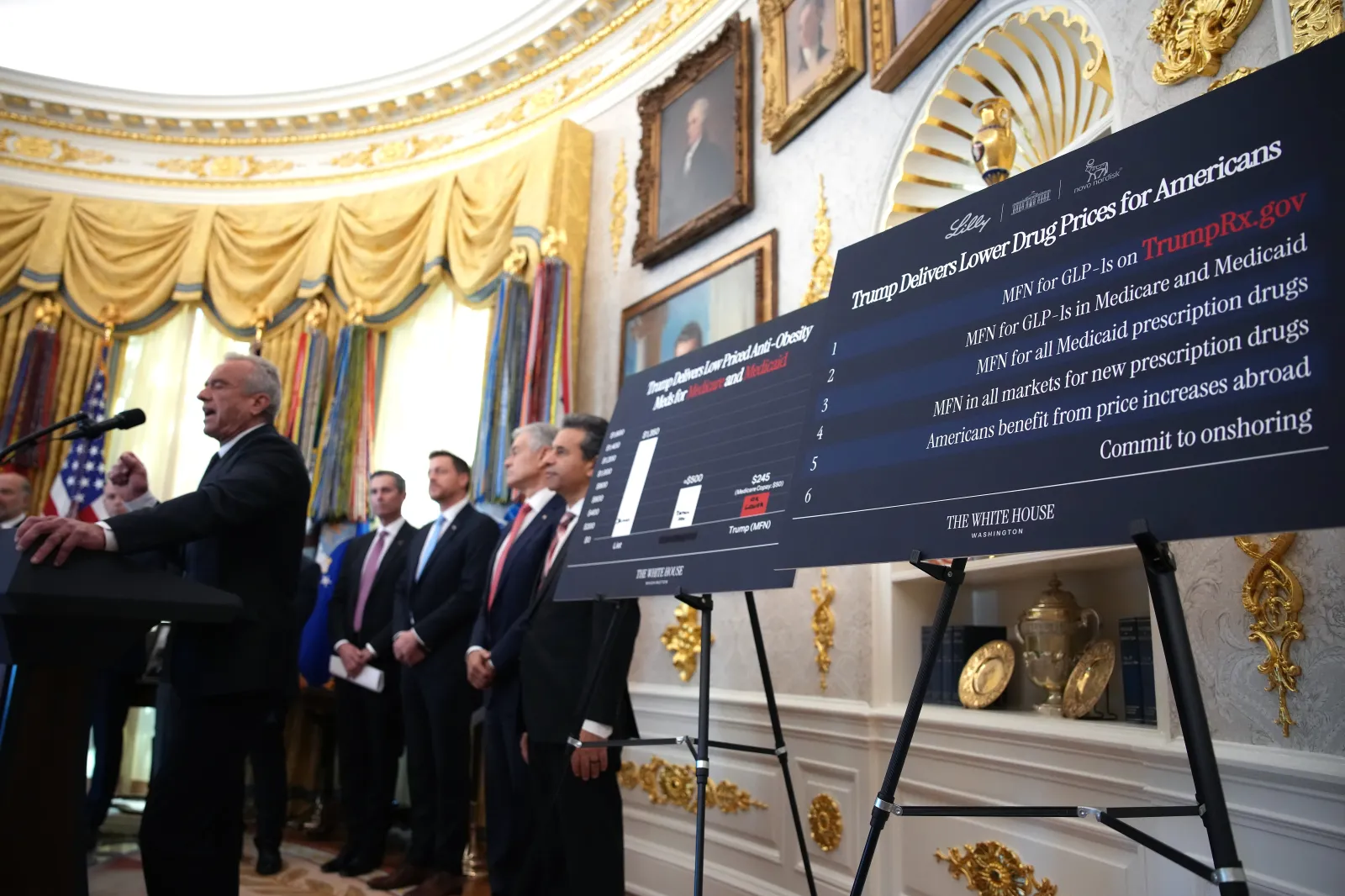
According to an official statement made by the CMS, on December 23, 2025, a new voluntary program—The Better Approaches to Lifestyle and Nutrition for Comprehensive hEalth (BALANCE)—is being rolled out, which will enable Medicare Part D plans and state Medicaid agencies to cover GLP-1 medications used for weight management and improvement of metabolic health.
Under the BALANCE model, the CMS will negotiate directly with pharmaceutical manufacturers to secure lower prices and uniform coverage terms for GLP‑1 medications, so Medicare and Medicaid beneficiaries get the same access and benefits regardless of where they are in the country.
The negotiations will focus on guaranteed pricing, potential price caps, standardized eligibility, coverage, and access criteria, and a mandatory inclusion of lifestyle support programs.
The aim is to make GLP-1 drugs more accessible for eligible Americans while containing costs for both patients and taxpayers. Participation in the BALANCE model will be voluntary for drug manufacturers, state Medicaid agencies, and Medicare Part D plans.
What People Are Saying
In its official statement, CMS Innovation Center Director Abe Sutton said: “The BALANCE Model will empower more Americans to live healthier lives by expanding access to GLP-1s that have shown to be a powerful tool against the development of diseases, such as diabetes, cardiovascular disease, and other metabolic conditions, which can negatively affect a person’s long-term health. Through this model, CMS will make GLP-1s more accessible for people with Medicare and Medicaid.”
CMS Administrator Dr. Mehmet Oz said: “Today’s announcement builds upon our historic Most Favored Nations drug pricing deals’ goal of democratizing access to weight-loss medication, which has been out of reach for so many in need. These actions further the administration’s bold plan to reform our country’s health systems and Make America Healthy Again. With the BALANCE Model, we’re pairing breakthrough science with healthy living to cut costs while empowering Americans to take control of their health.”
What Happens Next
BALANCE will launch in Medicaid as early as May 2026 and in Medicare Part D in January 2027. CMS is also planning to run a temporary Medicare GLP‑1 payment program, starting July 2026, to serve as a bridge to the BALANCE Model for Medicare members.
During this short-term program, eligible Medicare Part D enrollees will pay $50 per month for GLP‑1 medications. Coverage under both the temporary program and the full BALANCE Model will follow criteria that the CMS has negotiated with manufacturers, including prior authorization requirements and participation by manufacturers and plan sponsors.
If they wish to participate in the BALANCE program, drug manufacturers must respond to the BALANCE ‘Request For Applications’ (RFA), and state Medicaid agencies and Medicare Part D sponsors must submit a Notice of Intent by January 8, 2026.