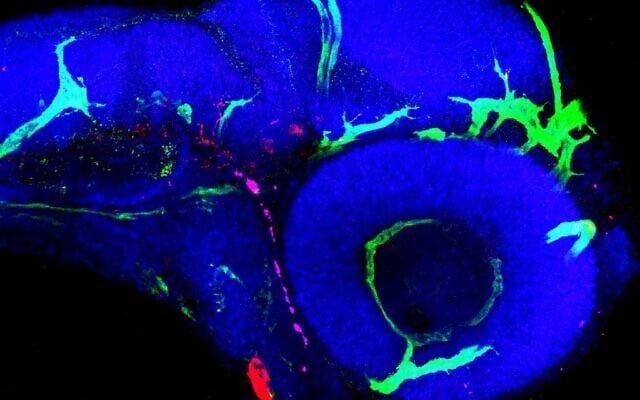
On the surface, humans and zebrafish couldn’t be more different. But they have an unexpected biological overlap that Weizmann Institute scientists and Sheba Medical Center physicians say helped them discover a promising new therapy for kaposiform lymphangiomatosis (KLA), a very rare but deadly genetic disease.
The joint project began when a nine-year-old boy came to Prof. Shoshana Greenberger’s Multidisciplinary Center for Vascular Anomalies at Sheba, suffering from severe shortness of breath. He was eventually diagnosed with KLA.
KLA affects the lymphatic system, and usually appears in children and teens. In a healthy body, the lymphatic system drains fluids and supports vital body functions. But patients with KLA have lymphatic vessels that grow abnormally large, leading to fluid buildup, especially in the chest. The disease can spread to vital organs and cause breathing problems and dangerous bleeding, and thus is often fatal.
Greenberger’s team traced the illness to a mutation in a gene called NRAS, which forces cells to grow uncontrollably. The physicians tried to use cancer drugs that block NRAS to save the young patient, but were unsuccessful, and the boy ultimately died.
Greenberger then approached Prof. Karina Yaniv of Weizmann’s Immunology and Regenerative Biology Department, who has been using zebrafish models to study how blood and lymphatic vessels form for over two decades.
Sign up for the Tech Israel Daily
and never miss Israel’s top tech stories
By signing up, you agree to the terms
The researchers teamed up and inserted the boy’s mutated NRAS gene into zebrafish embryos, creating fish that developed KLA-like symptoms.
Illustrative image of Zebrafish (Maksim Kazakov; iStock by Getty Images)
The scientists then identified two novel treatments that reversed the disease symptoms in the fish, and also successfully worked in the boy’s own cells in the lab.
The results of their findings were recently published in the peer-reviewed Journal of Experimental Medicine. Taking part in the collaborative experiment were Dr. Ivan Bassi, a postdoctoral fellow in Yaniv’s lab who created the zebrafish model of the human disease, and Amani Jabali, a graduate student at Weizmann.
“There is a huge need for treatments for these rare diseases, and we don’t have many options,” Yaniv told The Times of Israel.
Genetic similarities to humans
Zebrafish, with their distinctive blue and silver stripes, are members of the minnow family of fish and measure a little less than five centimeters (two inches) long. They can be found in home aquariums — as well as research labs. A mother can lay hundreds of transparent eggs at a time, which hatch within several days.
Zebrafish came into awareness of the scientific community in 1981 when American biologist George Streisinger bought a couple at a pet store in Oregon and began to do experiments with them, finding them useful as test subjects.
Yaniv explained the advantages of using these fish for biomedical research.
“Because they are transparent, we can basically follow their development,” she said. “It’s easy to do genetic manipulations with them. They are much cheaper and easier to grow than mice, and in terms of ethical regulations, it is much easier to work with fish than with large animals.”
A fluorescent image of a Zebrafish embryo. The blood vessels are green and the Trh neurons are magenta. (Courtesy/Deodatta Gajbhiye)
In their academic paper, “The not-so-long history of zebrafish research in Israel,” the authors write that the first zebrafish-focused laboratory was introduced at Tel Aviv University in 2001. Since then, more zebrafish research groups have been established in Israel, along with biomedical companies now utilizing this model.
In the lab, Yaniv said the team inserted the mutated human gene taken from the cells of Greenberger’s patient into tiny zebrafish embryos. The challenge was to ensure that the mutated gene was expressed in the fish’s lymphatic vessels alone and nowhere else in the body, as it is in human disease. Once this was achieved, the embryos developed lymphatic abnormalities that bore a remarkable resemblance to those of human KLA patients.
Dr. Ivan Bassi, a postdoctoral fellow in Prof. Karina Yaniv’s lab at the Weizmann Institute, created the zebrafish model of the human disease. (Courtesy)
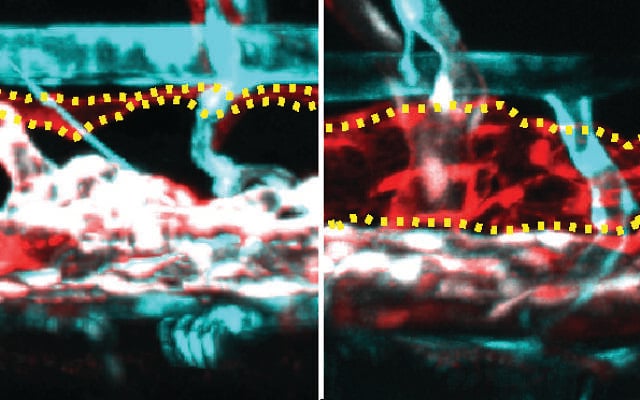
The scientists measured how the main lymphatic vessel of the embryos became greatly distorted, causing their hearts to become dilated. Further examination confirmed that these embryos shared key features with human KLA patients, including enlarged lymphatic vessels and swelling around the heart.
Bassi and his lab colleagues then deciphered previously unknown aspects of the NRAS mutated gene mechanism. In healthy cells, NRAS triggers cell division only when activated by a signal. In KLA, the mutated NRAS is stuck in the “on” position, causing lymphatic cells to divide and grow uncontrollably.
“It was an amazing moment,” Yaniv said. “Just by looking at these mutant embryos, I knew we were on the right track.”
Five-day-old zebrafish embryos viewed under a microscope. On top is a regular embryo. At the bottom is one that carries the mutated NRAS gene from a KLA patient. In the mutant, the main lymphatic vessel is abnormally enlarged. (Courtesy/Weizmann Institute)
Using an AI-based algorithm, the scientists outlined the entire body of the larval fish and measured its area after exposure to each drug. Because the mutant fish had enlarged hearts, their total body area was significantly greater than normal.
The team then screened about 150 existing drugs already approved for other uses. Two drugs reversed the KLA-like symptoms in the zebrafish model, with the ballooned heart and main lymphatic vessel shrinking back to their normal size and shape.
Under the microscope
The zebrafish embryos enabled the scientists to test potential drugs on living organisms, and not just isolated proteins or cells. But the tricky part was figuring out how to get each fish into exactly the right position under a microscope to capture consistent images and assess the effects of treatments.
Working with collaborators, the team designed an automated system in which each embryo was gently dropped into a precise slit under the microscope, where it was then photographed.
Lymphatic system of zebrafish embryos viewed under a microscope. On the right, the main lymph vessel, marked by a yellow dotted line, is abnormally enlarged in the mutant embryo compared to the regular one (left). The arteries are in turquoise. (Courtesy/Weizmann Institute)
To test whether these drugs might help treat the human disease, the team also applied them to lymphatic cells from Greenberger’s KLA patient. The two compounds had a striking effect. Importantly, both drugs have a better safety profile than the cancer drugs that physicians use to treat KLA today, with fewer side effects.
“We hope a clinical trial will be launched soon to evaluate these drugs in patients,” said Greenberger of Sheba. “Since KLA is a rare disease, we will work toward creating a multi-center collaboration to bring together enough participants.”
Meanwhile, Yaniv continues her work at Weizmann using zebrafish to investigate lymphatic disorders. During Israel’s war with Iran in June, an Iranian ballistic missile directly hit the Weizmann Institute, destroying 45 labs in two buildings and hundreds of research projects. But in Yaniv’s lab, only a few windows were shattered.
“We were very lucky,” she said.
Damage to the Weizmann Institute of Science from a June 14, 2025, Iranian missile strike in Rehovot, pictured on June 19, 2025. (AP Photo/Maya Alleruzzo)
In October 2024, the European Research Council (ERC) awarded Yaniv a Synergy Grant, which is part of the EU’s Horizon Europe research and innovation program.
A proposal to partially bar Israel from the Horizon program in response to allegations of starvation in Gaza did not pass in July, but the vote could be raised again later this month.
“I trust that people use their scientific common sense and not their political one when they judge science,” Yaniv said.