Molecular Diagnostics In Pharmacogenomics Market Analysis – Size, Share, and Forecast Outlook 2025 to 2035
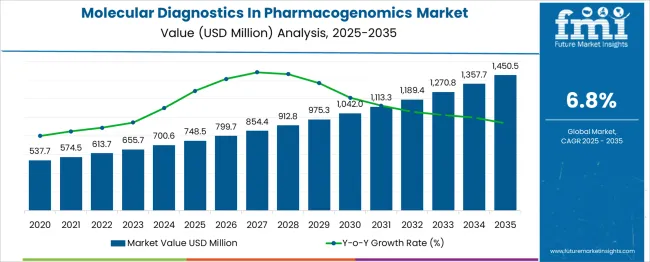
The global molecular diagnostics in pharmacogenomics market is projected to grow from USD 748.5 million in 2025 to approximately USD 1,450.5 million by 2035, recording an absolute increase of USD 702.0 million over the forecast period. This translates into a total growth of 93.8%, with the market forecast to expand at a compound annual growth rate (CAGR) of 6.8% between 2025 and 2035. The market size is expected to grow by nearly 1.94X during the same period, supported by increasing adoption of personalized medicine approaches, growing awareness about drug-gene interactions, and expanding applications in clinical decision-making for optimal therapeutic outcomes.
Between 2025 and 2030, the molecular diagnostics in pharmacogenomics market is projected to expand from USD 748.5 million to USD 1,025.7 million, resulting in a value increase of USD 277.2 million, which represents 39.5% of the total forecast growth for the decade. This phase of growth will be shaped by increasing integration of pharmacogenomic testing in routine clinical practice, expanding reimbursement coverage for genetic testing, and growing pharmaceutical industry adoption for drug development and clinical trials. Healthcare providers are increasingly recognizing the value of pharmacogenomic testing in reducing adverse drug reactions and optimizing therapeutic efficacy.
Quick Stats for Molecular Diagnostics In Pharmacogenomics Market
Molecular Diagnostics In Pharmacogenomics Market Value (2025): USD 748.5 million
Molecular Diagnostics In Pharmacogenomics Market Forecast Value (2035): USD 1,450.5 million
Molecular Diagnostics In Pharmacogenomics Market Forecast CAGR: 6.8%
Leading Technology in Molecular Diagnostics In Pharmacogenomics Market : PCR (38.1%)
Key Growth Regions in Molecular Diagnostics In Pharmacogenomics Market : North America, Europe, and Asia Pacific
Key Players in Molecular Diagnostics In Pharmacogenomics Market :Thermo Fisher Scientific, Roche Diagnostics, QIAGEN, Illumina Inc, Agilent Technologies, Abbott Molecular, Genomind, OneOme, Myriad Genetics, Invitae
Molecular Diagnostics In Pharmacogenomics Market Key Takeaways
Metric
Value
Estimated Value in (2025E)
USD 748.5 million
Forecast Value in (2035F)
USD 1,450.5 million
Forecast CAGR (2025 to 2035)
6.8%
From 2030 to 2035, the market is forecast to grow from USD 1,025.7 million to USD 1,450.5 million, adding another USD 424.8 million, which constitutes 60.5% of the ten-year expansion. This period is expected to be characterized by advancement in next-generation sequencing technologies, integration of artificial intelligence in data interpretation, and development of comprehensive pharmacogenomic panels. The growing priority on precision medicine and value-based healthcare will drive demand for sophisticated molecular diagnostic solutions that can guide personalized treatment strategies.
Between 2020 and 2025, the molecular diagnostics in pharmacogenomics market experienced steady expansion, driven by increasing awareness about genetic factors influencing drug response and growing adoption of companion diagnostics. The market developed as healthcare systems recognized the potential of pharmacogenomics to reduce healthcare costs through improved drug efficacy and reduced adverse events. Clinical guidelines began incorporating pharmacogenomic testing recommendations for specific drug-gene pairs, particularly in oncology and psychiatry therapeutic areas.
Why the Molecular Diagnostics In Pharmacogenomics Market is Growing?
Market expansion is being supported by the increasing recognition of genetic variability in drug metabolism and response among different patient populations. Healthcare providers are increasingly adopting pharmacogenomic testing to optimize drug selection and dosing, particularly for medications with narrow therapeutic windows or high risk of adverse reactions. The growing body of clinical evidence demonstrating improved patient outcomes through pharmacogenomic-guided therapy is driving acceptance among clinicians and payers.
The advancement in molecular diagnostic technologies, particularly next-generation sequencing and high-throughput genotyping platforms, is making pharmacogenomic testing more accessible and cost-effective. Decreasing costs of genetic testing combined with faster turnaround times are enabling broader implementation in clinical settings. The development of comprehensive multi-gene panels that can assess multiple drug-gene interactions simultaneously is creating value propositions for healthcare systems seeking to implement precision medicine approaches across various therapeutic areas.
Segmental Analysis
The market is segmented by product outlook, therapeutic area outlook, and technology outlook. By product outlook, the market is divided into kits and assays, reagents, and services. Based on therapeutic area outlook, the market is categorized into oncology (including lung cancer, breast cancer, colorectal cancer, cervical cancer, and others), neurological disorders, cardiovascular disease, immunological disorders, infectious diseases, and others. In terms of technology outlook, the market is segmented into PCR, microarray, sequencing, and others.
By Product Outlook, Kits and Assays Segment Accounts for 48.1% Market Share
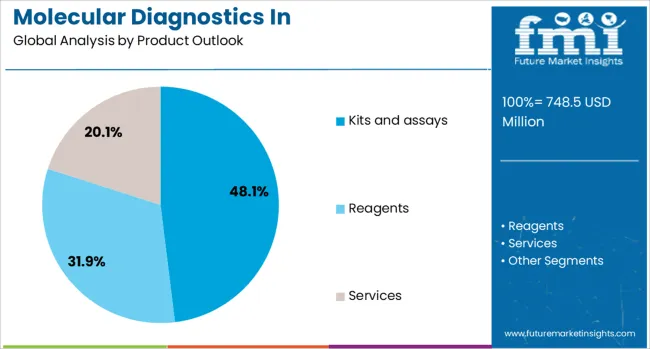
The kits and assays segment is projected to account for 48.1% of the molecular diagnostics in pharmacogenomics market in 2025, establishing its position as the dominant product category. This prominence reflects the essential role of ready-to-use testing solutions in clinical laboratories and healthcare facilities seeking to implement pharmacogenomic testing efficiently. Kits and assays provide standardized, validated methodologies that ensure consistent results across different testing environments, which is crucial for clinical decision-making.
The segment benefits from continuous innovation in assay design, including multiplex capabilities that allow simultaneous detection of multiple genetic variants relevant to drug metabolism. FDA-cleared and CE-marked kits provide regulatory compliance and clinical validation that healthcare providers require for routine implementation. The availability of both targeted single-gene assays and comprehensive panel-based solutions allows laboratories to select products appropriate for their testing volumes and clinical needs. As pharmacogenomic testing becomes more integrated into standard care protocols, the demand for reliable, user-friendly kits and assays continues to drive this segment’s growth.
By Therapeutic Area Outlook, Oncology Segment Accounts for 33.4% Market Share
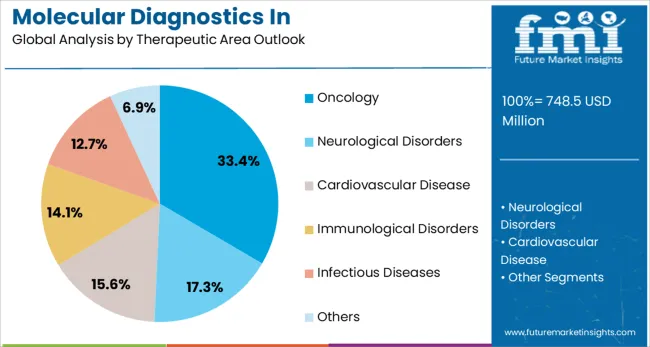
Oncology is projected to represent 33.4% of molecular diagnostics in pharmacogenomics demand in 2025, reflecting its position as the leading therapeutic application area. The complexity of cancer treatment and the high stakes involved in therapeutic selection make pharmacogenomic testing particularly valuable in oncology settings. Companion diagnostics for targeted therapies have become standard practice, with genetic testing required or recommended for numerous cancer drugs to identify patients most likely to benefit from specific treatments.
The segment encompasses various cancer types including lung cancer, breast cancer, colorectal cancer, and cervical cancer, each with specific pharmacogenomic markers guiding treatment decisions. The growing pipeline of targeted therapies and immunotherapies further drives demand for molecular diagnostic testing to stratify patients and predict treatment response. The pharmacogenomic testing helps identify patients at risk for severe toxicity from chemotherapy agents, enabling dose adjustments or alternative treatment selections that improve patient safety and quality of life.
By Technology Outlook, PCR Segment Accounts for 38.1% Market Share
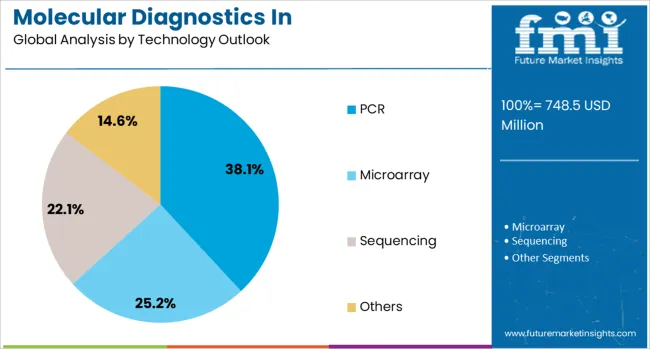
The PCR (Polymerase Chain Reaction) technology is forecasted to contribute 38.1% of the molecular diagnostics in pharmacogenomics market in 2025, maintaining its position as the most widely adopted testing platform. PCR’s dominance stems from its proven reliability, cost-effectiveness, and widespread availability in clinical laboratories worldwide. The technology offers excellent sensitivity and specificity for detecting specific genetic variants relevant to drug metabolism, making it ideal for targeted pharmacogenomic testing.
Real-time PCR and digital PCR variants provide quantitative capabilities essential for certain pharmacogenomic applications, while the development of multiplex PCR assays enables simultaneous detection of multiple variants in a single reaction. The technology’s relatively simple workflow and fast turnaround times make it suitable for routine clinical use, particularly for high-volume testing of common pharmacogenomic markers. As laboratories seek to balance comprehensive testing capabilities with operational efficiency, PCR-based solutions continue to serve as the backbone of pharmacogenomic testing programs.
What are the Drivers, Restraints, and Key Trends of the Molecular Diagnostics In Pharmacogenomics Market ?
The molecular diagnostics in pharmacogenomics market is advancing steadily due to increasing adoption of precision medicine approaches and growing evidence supporting pharmacogenomic-guided therapy. The market faces challenges including limited reimbursement coverage, lack of standardized clinical guidelines, and need for healthcare provider education. Innovation in testing technologies and expanding clinical applications continue to influence market development and adoption patterns.
Integration of Artificial Intelligence and Machine Learning in Data Interpretation
The incorporation of AI and machine learning algorithms is revolutionizing the interpretation of pharmacogenomic data, enabling more sophisticated analysis of complex drug-gene interactions. These technologies help identify patterns and associations that might be missed through traditional analysis methods, improving the clinical utility of test results. Advanced decision support systems are being developed to help clinicians translate genetic information into actionable treatment recommendations, addressing one of the key barriers to pharmacogenomic implementation.
Development of Point-of-Care Testing Solutions
The emergence of point-of-care molecular diagnostic platforms is making pharmacogenomic testing more accessible in various healthcare settings. These rapid testing solutions enable real-time therapeutic decision-making, particularly valuable in acute care situations where drug selection timing is critical. Miniaturized testing devices and simplified workflows are reducing the infrastructure requirements for pharmacogenomic testing, potentially expanding access to underserved populations and resource-limited settings.
Analysis of Molecular Diagnostics In Pharmacogenomics Market by Key Country
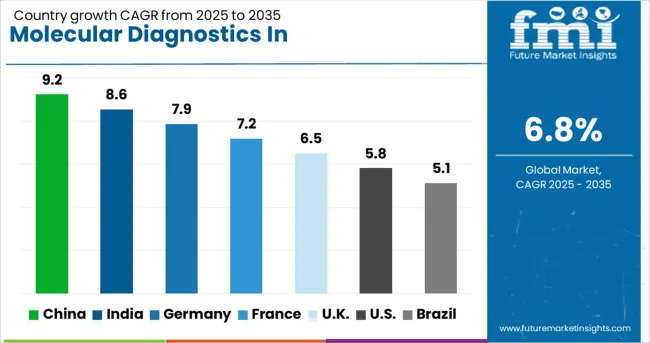
Country
CAGR (2025-2035)
China
9.2%
India
8.6%
Germany
7.9%
France
7.2%
UK
6.5%
USA
5.8%
Brazil
5.1%
The molecular diagnostics in pharmacogenomics market is experiencing varied growth globally, with China leading at a 9.2% CAGR through 2035, driven by massive healthcare infrastructure investments, growing precision medicine initiatives, and expanding clinical adoption of pharmacogenomic testing. India follows at 8.6%, supported by increasing healthcare expenditure, growing awareness of personalized medicine, and expanding diagnostic laboratory networks. Germany shows strong growth at 7.9%, prioritizing integration of pharmacogenomics into clinical practice and comprehensive reimbursement policies. France records 7.2%, focusing on national genomic medicine programs and coordinated implementation strategies. The UK demonstrates 6.5% growth, prioritizing NHS-led initiatives and clinical guideline development.
The report covers an in-depth analysis of 40+ countries; seven top-performing countries are highlighted below.
China Leads Asian Market Growth with Precision Medicine Initiatives
Revenue from molecular diagnostics in pharmacogenomics in China is projected to exhibit strong growth with a CAGR of 9.2% through 2035, driven by government-led precision medicine initiatives and substantial investments in genomic research infrastructure. The country’s Healthy China 2030 strategy focus personalized medicine as a key component of healthcare modernization. Major hospitals and research institutions are establishing pharmacogenomic testing programs to improve drug efficacy and reduce adverse drug reactions among the Chinese population.
Government funding for precision medicine research and clinical implementation is accelerating adoption of pharmacogenomic testing across tier-1 and tier-2 cities throughout the country.
Collaboration between international diagnostic companies and local partners is enhancing technology transfer and expanding access to advanced molecular diagnostic platforms nationwide.
India Demonstrates Strong Market Potential with Growing Healthcare Infrastructure
Revenue from molecular diagnostics in pharmacogenomics in India is expanding at a CAGR of 8.6%, supported by increasing healthcare awareness, rising incidence of chronic diseases requiring precision therapeutic approaches, and growing private healthcare sector investments. The country’s large patient population and genetic diversity create significant opportunities for pharmacogenomic research and clinical application. Both public and private healthcare institutions are beginning to integrate pharmacogenomic testing into specialty care areas.
Rising medical tourism and demand for advanced diagnostic services are driving adoption of pharmacogenomic testing in premium healthcare facilities across major metropolitan areas.
Growing number of clinical laboratories obtaining international accreditations is improving testing quality and reliability, supporting broader acceptance of pharmacogenomic testing among healthcare providers.
United States Maintains Market Leadership with Established Infrastructure
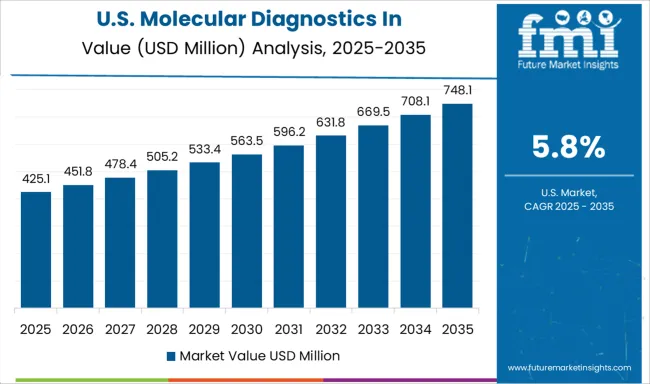
Demand for molecular diagnostics in pharmacogenomics in the USA is projected to grow at a CAGR of 5.8%, supported by well-established precision medicine programs, comprehensive clinical guidelines, and increasing payer coverage for pharmacogenomic testing. American healthcare institutions lead in clinical implementation of pharmacogenomics, with many major medical centers offering routine testing for drug-gene interactions. The market benefits from strong regulatory frameworks and extensive clinical evidence supporting test utility.
Expanding coverage by Medicare and private insurers for specific pharmacogenomic tests is improving patient access and driving market growth across diverse patient populations.
Integration of pharmacogenomic data into electronic health records and clinical decision support systems is facilitating routine use of genetic information in prescribing decisions.
Germany Anchors European Growth with Comprehensive Healthcare Integration
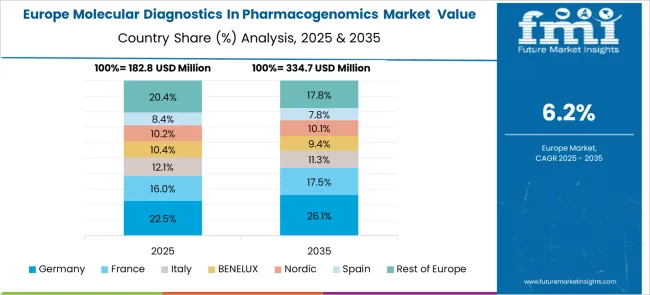
Revenue from molecular diagnostics in pharmacogenomics in Germany is projected to grow at a CAGR of 7.9% through 2035, driven by the country’s advanced healthcare system and strong focus on evidence-based medicine. German healthcare providers are increasingly adopting pharmacogenomic testing as part of comprehensive patient care strategies, particularly in oncology and psychiatry. The market benefits from favorable reimbursement policies and well-developed molecular diagnostic infrastructure.
National initiatives promoting personalized medicine and biomarker-driven therapy are supporting systematic implementation of pharmacogenomic testing in clinical practice.
Collaboration between academic medical centres, diagnostic laboratories, and pharmaceutical companies is advancing research and clinical validation of pharmacogenomic applications.
France Advances Market with National Genomic Medicine Programs
Revenue from molecular diagnostics in pharmacogenomics in France is projected to grow at a CAGR of 7.2% through 2035, supported by the France Genomic Medicine 2025 plan and coordinated efforts to integrate pharmacogenomics into routine healthcare. French medical institutions are establishing specialized pharmacogenomic services and developing national guidelines for test implementation across various therapeutic areas.
Government investment in genomic medicine infrastructure and professional education is building capacity for widespread pharmacogenomic testing implementation throughout the healthcare system.
Strong pharmaceutical industry presence and clinical research capabilities are supporting development and validation of new pharmacogenomic biomarkers and testing applications.
United Kingdom Strengthens Position with NHS-Led Initiatives
Revenue from molecular diagnostics in pharmacogenomics in the UK is projected to grow at a CAGR of 6.5% through 2035, driven by NHS England’s commitment to embedding pharmacogenomics in routine care and the 100,000 Genomes Project legacy. British healthcare institutions are developing implementation frameworks and clinical pathways for pharmacogenomic testing across multiple specialties.
NHS commissioning of pharmacogenomic testing for specific indications is establishing precedents for routine clinical use and supporting market growth across public healthcare settings.
Academic leadership in pharmacogenomic research and clinical implementation is generating evidence and best practices that facilitate broader adoption throughout the healthcare system.
Brazil Expands Market with Growing Healthcare Modernization
Revenue from molecular diagnostics in pharmacogenomics in Brazil is projected to grow at a CAGR of 5.1% through 2035, supported by healthcare system improvements, increasing prevalence of chronic diseases, and growing awareness of personalized medicine benefits. Brazilian healthcare providers are beginning to adopt pharmacogenomic testing, particularly in private healthcare settings and specialized treatment centers.
Expanding private healthcare sector and medical insurance coverage for genetic testing are improving access to pharmacogenomic services among middle and upper-income populations.
Collaboration with international diagnostic companies and technology transfer initiatives are enhancing local testing capabilities and supporting market development across major urban centers.
Competitive Landscape of Molecular Diagnostics In Pharmacogenomics Market
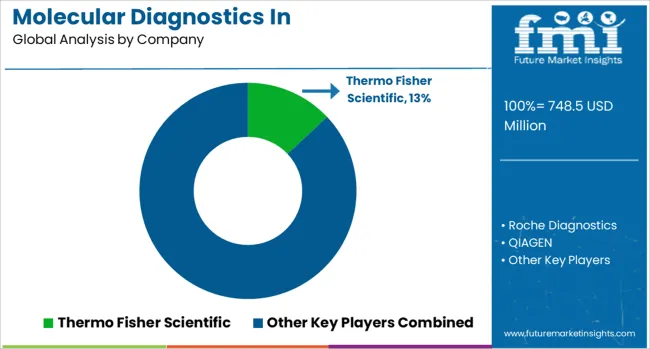
The molecular diagnostics in pharmacogenomics market is characterized by competition among established diagnostic companies, specialized genetic testing laboratories, and emerging technology providers. Companies are investing in platform development, bioinformatics capabilities, clinical validation studies, and market access strategies to deliver comprehensive pharmacogenomic testing solutions. Test menu expansion, technological innovation, and clinical utility demonstration are central to strengthening market positions and driving adoption.
Thermo Fisher Scientific, USA-based, leads the market with 13% global value share, offering comprehensive molecular diagnostic platforms and pharmacogenomic testing solutions with strong research and clinical market presence. Roche Diagnostics provides integrated diagnostic systems with extensive pharmacogenomic test menus and companion diagnostic expertise. QIAGEN delivers sample-to-insight solutions with specialized pharmacogenomic content and bioinformatics capabilities. Illumina, Inc. focuses on next-generation sequencing platforms enabling comprehensive pharmacogenomic profiling.
Agilent Technologies provides molecular diagnostic instruments and reagents supporting pharmacogenomic testing workflows. Abbott Molecular offers automated molecular diagnostic systems with pharmacogenomic testing capabilities. Genomind specializes in psychiatric pharmacogenomics with proprietary testing panels and clinical decision support. OneOme focuses on comprehensive pharmacogenomic testing with medication management tools. Myriad Genetics provides specialized pharmacogenomic tests with strong clinical validation. Invitae delivers pharmacogenomic testing with integrated clinical decision support and medication management platforms.
Key Players in the Molecular Diagnostics In Pharmacogenomics Market
Thermo Fisher Scientific
Roche Diagnostics
QIAGEN
Illumina, Inc.
Agilent Technologies
Abbott Molecular
Genomind
OneOme
Myriad Genetics
Invitae
Scope of the Report
Items
Values
Quantitative Units (2025)
USD 748.5 million
Product Outlook
Kits and assays, Reagents, Services
Therapeutic Area Outlook
Oncology (Lung Cancer, Breast Cancer, Colorectal Cancer, Cervical Cancer, Others), Neurological Disorders, Cardiovascular Disease, Immunological Disorders, Infectious Diseases, Others
Technology Outlook
PCR, Microarray, Sequencing, Others
Regions Covered
North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa
Countries Covered
United States, Canada, United Kingdom, Germany, France, China, Japan, South Korea, India, Brazil, Australia and 40+ countries
Key Companies Profiled
Thermo Fisher Scientific, Roche Diagnostics, QIAGEN, Illumina Inc., Agilent Technologies, Abbott Molecular, Genomind, OneOme, Myriad Genetics, and Invitae (formerly YouScript)
Additional Attributes
Market share analysis by technology platform and therapeutic application, regional adoption trends, competitive landscape assessment, reimbursement scenarios across key markets, integration with electronic health records and clinical decision support systems, regulatory pathway analysis for pharmacogenomic tests, clinical utility evidence and health economic outcomes

