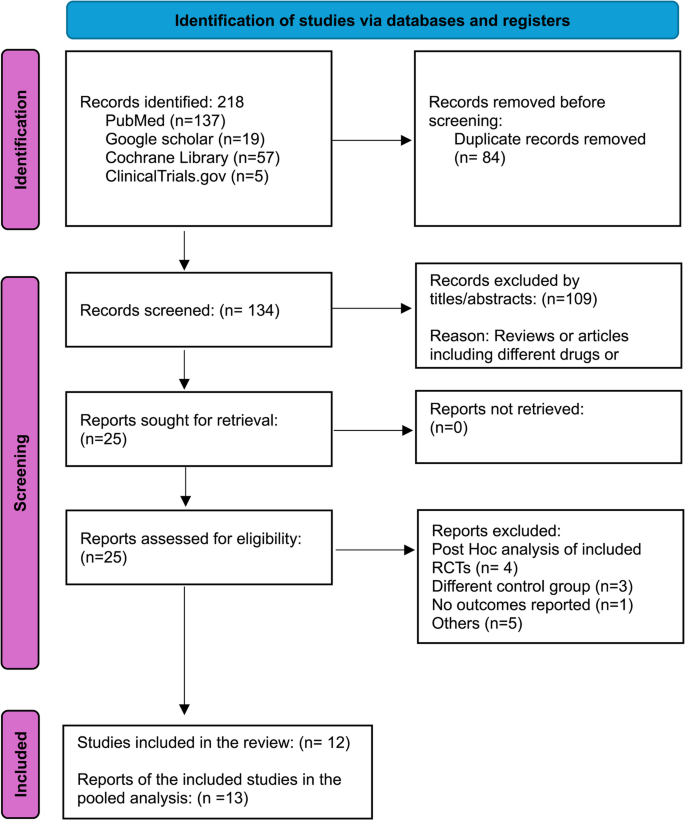
A total of 218 records were identified through database searches, with 134 remaining after duplicate removal. After screening and full-text assessment, 12 studies with 13 reports met the inclusion criteria and were included in the pooled analysis [8, 9, 14, 15, 20,21,22,23,24,25,26,27], while one study was excluded due to a lack of reported outcomes [28]. (Fig. 1).
PRISMA flow diagram illustrating the study selection process
Summary of included studies
This meta-analysis included 12 studies with 13 reports [8, 9, 14, 15, 20,21,22,23,24,25,26,27], comprising RCTs and retrospective cohort studies, published between 2005 and 2024, with a total of 5,728 participants. Among these, 2,287 received clazosentan, 2,270 were treated with fasudil hydrochloride, and 1,171 were assigned to the placebo group. The study population consisted of 1,727 males and 3,843 females. Clazosentan was administered at doses Ranging from 1 mg/h to 15 mg/h for a duration of 14 to 15 days, with treatment initiation occurring within 24 to 96 h. The mean age of participants varied between 45 and 80.2 years. Aneurysmal treatment modalities included surgical clipping, endovascular coiling, or a combination of both. The analysis encompassed two Phase II trials, six Phase III trials, and five retrospective cohort studies. Clazosentan was evaluated in comparison to placebo or fasudil hydrochloride, offering a comprehensive assessment of its efficacy and safety across diverse clinical settings. Table 1.
Table 1 Study and baseline characteristicsQuality assessment
The quality assessment of the included studies was conducted using the Cochrane Risk of Bias tool for RCTs and the NOS for cohort studies. The RCTs demonstrated a generally low risk of bias across key domains such as random sequence generation, allocation concealment, and blinding. (Supplementary Fig. 1) For the cohort studies, the methodological quality was high, with total scores Ranging from 7 to 8 out of 9, indicating a low risk of bias. All studies had strong selection criteria, adequate follow-up, and well-defined outcome assessments. (Supplementary Table 2).
Vasospasm-related new cerebral infarct
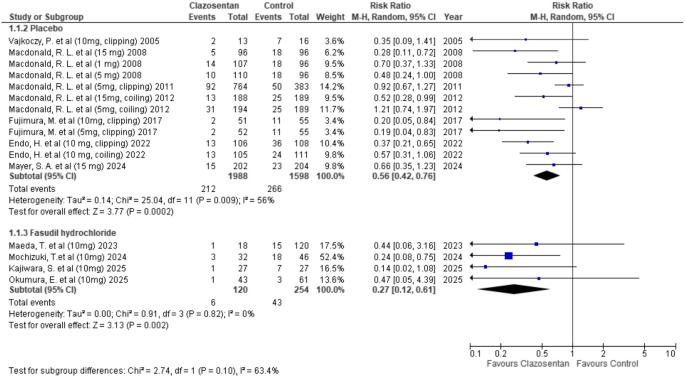
Clazosentan was associated with a significantly lower risk of vasospasm-related new cerebral infarct compared to placebo (RR: 0.56; 95% CI: 0.42–0.76; P = 0.0002; I² = 56%). Similarly, compared to Fasudil hydrochloride, Clazosentan also demonstrated a reduced risk (RR: 0.27; 95% CI: 0.12–0.61; P = 0.002; I² = 0%). (Fig. 2)
Forest plot depicting the effect of Clazosentan on the risk of vasospasm-related new cerebral infarct compared to placebo and Fasudil hydrochloride
Vasospasm-related DIND
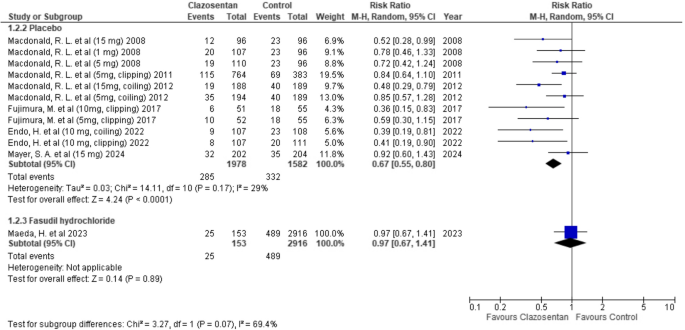
Clazosentan significantly reduced the risk of vasospasm-related DIND compared to placebo (RR: 0.67; 95% CI: 0.55–0.80; P < 0.0001; I² = 29%). However, there was no significant difference when compared to Fasudil hydrochloride (RR: 0.97; 95% CI: 0.67–1.41; P = 0.89). (Fig. 3)
Forest plot depicting the effect of Clazosentan on the risk of vasospasm-related DIND compared to placebo and Fasudil hydrochloride
Incidence of vasospasm
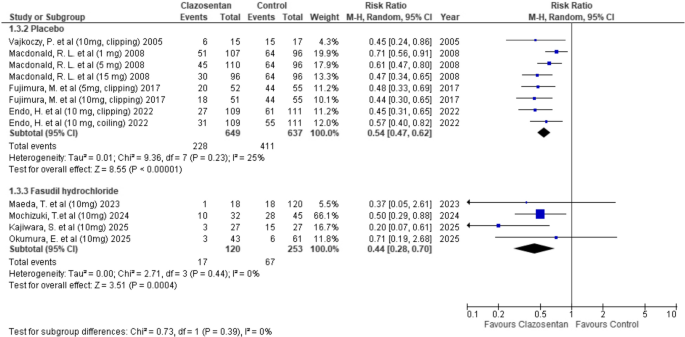
Clazosentan significantly reduced the incidence of vasospasm compared to placebo (RR: 0.54; 95% CI: 0.47–0.62; P < 0.00001; I² = 25%). A similar reduction was observed when compared to Fasudil hydrochloride (RR: 0.44; 95% CI: 0.28–0.70; P = 0.0004; I² = 0%). (Fig. 4)
Forest plot depicting the effect of Clazosentan on the risk of vasospasm compared to placebo and Fasudil hydrochloride
Rescue therapy
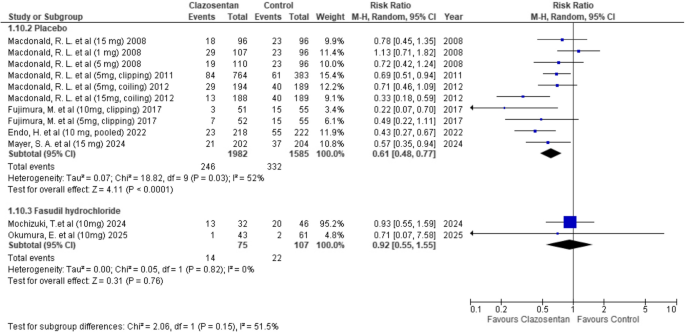
Clazosentan significantly reduced the need for rescue therapy compared to placebo (RR: 0.61; 95% CI: 0.48–0.77; P < 0.0001; I² = 52%). However, there was no significant difference when compared to Fasudil hydrochloride (RR: 0.92; 95% CI: 0.55–1.55; P = 0.76; I² = 0%). (Fig. 5)
Forest plot depicting the effect of Clazosentan on the risk of requiring rescue therapy compared to placebo and Fasudil hydrochloride
GOSE < 4
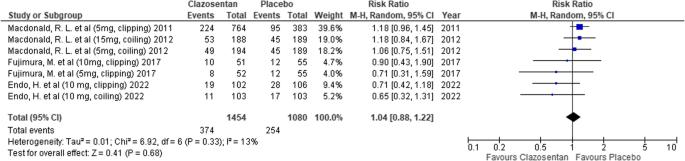
Clazosentan showed no significant difference in the risk of GOSE < 4 compared to placebo (RR: 1.04; 95% CI: 0.88–1.22; P = 0.68; I² = 13%). (Fig. 6)
Forest plot depicting the effect of Clazosentan on the risk of a Glasgow Outcome Scale–Extended (GOSE) score < 4 compared to placebo
Mortality
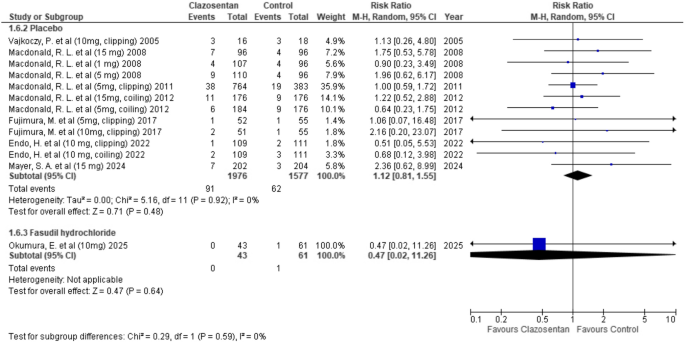
Clazosentan showed no significant difference in mortality compared to placebo (RR: 1.12; 95% CI: 0.81–1.55; P = 0.48; I² = 0%) and similarly, no significant difference was observed when compared to fasudil hydrochloride (RR: 0.47; 95% CI: 0.02–11.26; P = 0.64). (Fig. 7)
Forest plot depicting the effect of Clazosentan on the risk of mortality compared to placebo and Fasudil hydrochloride
Anemia
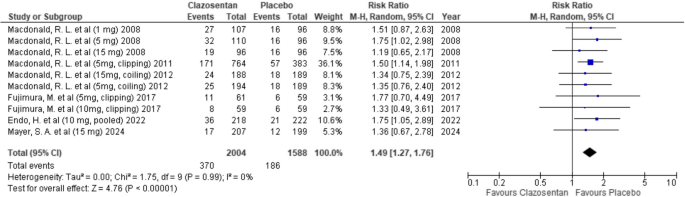
Clazosentan was associated with a significantly increased risk of anemia compared to placebo (RR: 1.49; 95% CI: 1.27–1.76; P < 0.00001; I² = 0%). (Fig. 8)
Forest plot depicting the effect of Clazosentan on the risk of anemia compared to placebo
Pleural effusion
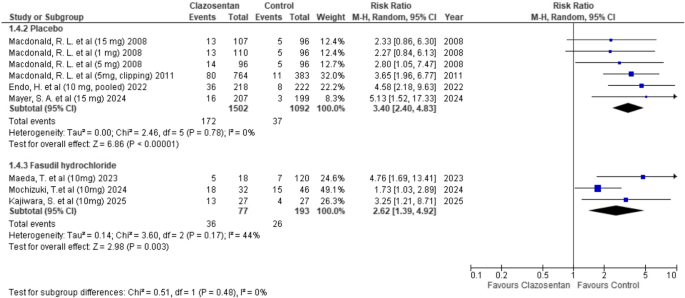
Clazosentan was associated with a significantly higher risk of pleural effusion compared to placebo (RR: 3.40; 95% CI: 2.40–4.83; P < 0.00001; I² = 0%). When compared to fasudil hydrochloride, the increased risk was statistically significant (RR: 2.62; 95% CI: 1.39–4.92; P = 0.003; I² = 44%). (Fig. 9)
Forest plot depicting the effect of Clazosentan on the risk of pleural effusion compared to placebo and Fasudil hydrochloride
Hypotension
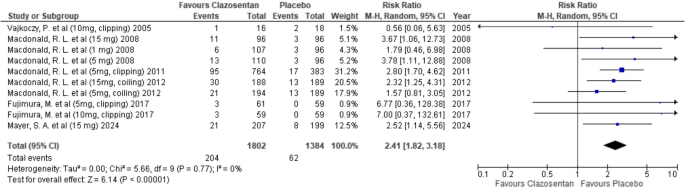
Clazosentan was associated with a significantly higher risk of hypotension compared to placebo (RR: 2.41; 95% CI: 1.82–3.18; P < 0.00001; I² = 0%). (Fig. 10)
Forest plot depicting the effect of Clazosentan on the risk of hypotension compared to placebo
Pulmonary edema
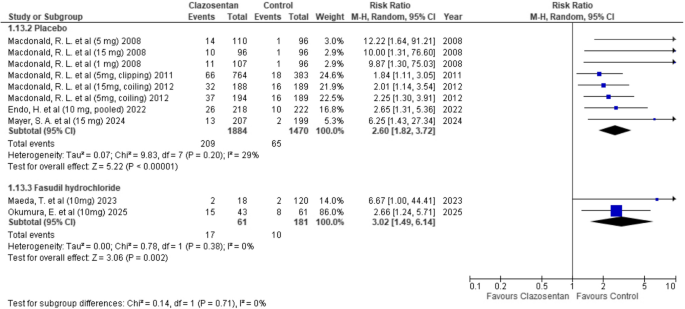
Clazosentan was associated with a significantly higher risk of pulmonary edema compared to placebo (RR: 2.60; 95% CI: 1.82–3.72; P < 0.00001; I² = 29%). When compared to fasudil hydrochloride, clazosentan showed a statistically significant increase in risk (RR: 3.02; 95% CI: 1.49–6.14; P = 0.002; I² = 0%). (Fig. 11)
Forest plot depicting the effect of Clazosentan on the risk of pulmonary edema compared to placebo and Fasudil hydrochloride
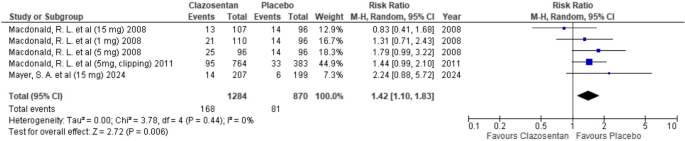
Pneumonia
Clazosentan was associated with a higher risk of pneumonia compared to placebo; however, the difference was not statistically significant (RR: 1.42; 95% CI: 1.10–1.83; P = 0.006; I² = 0%). (Fig. 12)
Forest plot depicting the effect of Clazosentan on the risk of pneumonia compared to placebo
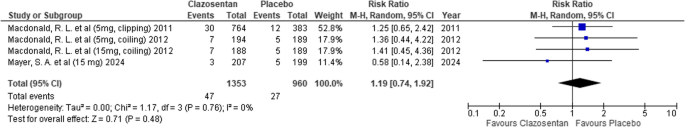
Cerebral hemorrhage
Clazosentan showed no significant difference in the risk of cerebral hemorrhage compared to placebo (RR: 1.19; 95% CI: 0.74–1.92; P = 0.48; I² = 0%). (Fig. 13)
Forest plot depicting the effect of Clazosentan on the risk of cerebral hemorrhage compared to placebo
Subgroup analysis by Clazosentan dosage (Supplementary table 3)
1: Vasospasm-Related New Cerebral Infarct: Lower at 10 mg/h and 15 mg/h; no significant difference at lower doses.
2: Vasospasm-Related DIND: Reduced at 5 mg/h, 10 mg/h, and 15 mg/h but not at 1 mg/h.
3: Vasospasm Incidence: Reduced at all doses, with the greatest reduction at 15 mg/h.
4: Rescue Therapy: Trend toward reduction, significant at 5 mg/h, 10 mg/h, and 15 mg/h.
5: GOSE < 4 & Mortality: No significant difference across all doses.
6: Adverse Events: Higher rates of anemia, hypotension, and pulmonary edema with increasing doses. No significant difference in cerebral hemorrhage.
Subgroup analysis by aneurysm treatment type (Supplementary table 4)
1: Vasospasm-Related New Cerebral Infarct: Lower in surgical clipping; no significant difference in endovascular coiling.
2: Vasospasm-Related DIND: Reduced in both surgical clipping and endovascular coiling.
3: Vasospasm Incidence: Reduced with surgical clipping but not with endovascular coiling.
4: Rescue Therapy: Trend toward reduction in both, significant only in endovascular coiling.
5: GOSE < 4 & Mortality: No significant difference in both surgical clipping and endovascular coiling.
6: Adverse Events: Anemia, hypotension, and pulmonary edema were more frequent with clazosentan in both surgical clipping and endovascular coiling. No significant difference in cerebral hemorrhage.