Our study demonstrates that CERT scores and Cer components are significantly associated with COPD prevalence and incidence. CERT1 and CERT2 showed significant associations with prevalent and incident COPD, though in most cases the significance was lost after adjusting for confounders, but these findings remain consistent with previous research linking ceramides to lung inflammation, emphysema, and tissue damage [22]. After stratification of the sexes among incident study groups, CERT2 showed stronger associations in men, particularly for incident COPD, suggesting its role in COPD pathophysiology. CERT1 and CERT2 performed better in predicting COPD incidents among smokers than non-smokers. Among the lipids and ratios, Cer (d18:1/24:1) emerged as a key biomarker positively associated with COPD prevalence, while higher PC (14:0/22:6) was inversely associated with COPD incidence, suggesting a potential protective role.
These findings are in agreement with a Korean cohort study that showed elevated Cer levels (d18:1/18:0) and Cer (d18:1/24:1) were associated with reduced FEV1 and prevalent COPD [23], indicating their involvement in the decline of lung function and disease onset. Though in our study, Cer (d18:1/18:0) was only significant in the unadjusted models. In contrast, Cer(d18:1/24:1) was significant in both models in the prevalence study, suggesting that its role in COPD development may be independent of the metabolic changes caused by smoking.
We found that the Cer (d18:1/18:0)/PC (14:0/22:6) ratio was associated with both prevalent and incident COPD, thus serving as a reliable general risk marker for the disease. However, Cer (d18:1/18:0)/Cer (d18:1/24:0) was specifically associated with incident COPD, indicating it may be a prognostic indicator for people at high risk of getting this disease. As a result of these findings, while Cer (d18:1/18:0)/PC (14:0/22:6) may help assess COPD risk generally, Cer (d18:1/18:0)/Cer (d18:1/24:0) might be better suited for predicting COPD incidence.
The findings of our studies are in accordance with those of previous studies suggesting that Cer accumulation in lung tissue contributes to apoptosis and emphysema progression [24]. These results underscore the potential of Cers as early biomarkers of COPD, serving as indicators of both disease presence and progression.
The findings of Mizumura et al. suggest that Cers contribute to cell death and autophagy (self-digestion) in patients with COPD. Following their findings, we evaluated Cer-to-PC ratios, such as Cer (d18:1/18:0)/PC (14:0/22:6), to assess whether they can provide more precise markers for tracking COPD progression and development [25]. These findings suggest the potential utility of Cers in COPD, highlighting the need for further research to validate these biomarkers for early diagnosis and personalized treatment strategies.
Sex-specific findings and their implications
According to our study, there are sex-based differences in the association between certain Cers and COPD. The association between CERT1, CERT2, and COPD was significant in men in unadjusted models. However, after adjustments, only CERT2, PC (14:0/22:6), Cer (d18:1/18:0)/Cer (d18:1/24:0), and (d18:1/18:0)/PC (14:0/22:6) remained significant, indicating a specific link to COPD risk in men. In the male group, CERT2 also performed a bit better in predicting COPD incidence than CERT1.
In contrast, among women, although initial unadjusted models showed significant associations, none of the CERT scores or components remained significant after adjustment. This discrepancy may partly reflect the smaller number of COPD cases in women (N = 56) compared to men (N = 149), resulting in lower statistical power for the adjusted models in women. The findings of this study are consistent with those of Ekroos et al., who reported that ceramides and other lipid biomarkers can distinguish cardiovascular and pulmonary disease risks, although these associations vary by sex, age, and other demographic factors. Their review highlights the need to standardize lipidomics to improve disease monitoring, as both our study and theirs illustrate the complex interplay of lipids in COPD and related diseases [26]. Evidence supporting systemic lipid peroxidation and oxidative stress in COPD further strengthens the notion of sex-specific variations in lipid profiles and disease outcomes [27, 28]. These results underscore the importance of developing tailored therapeutic approaches that consider sex differences, as men and women may experience distinct patterns of disease progression.
Impact of smoking on ceramide metabolism
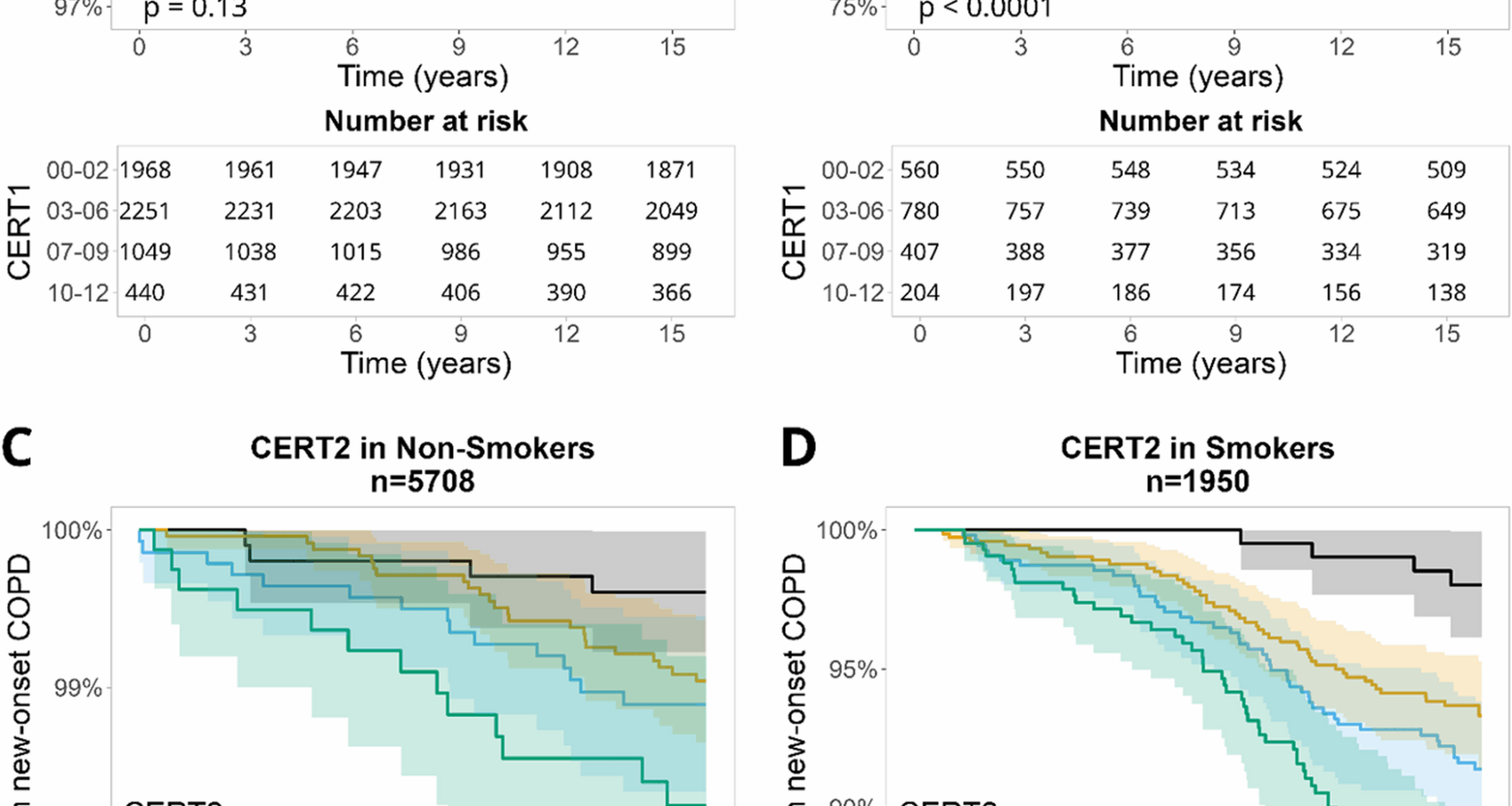
Kaplan–Meier survival curves indicated that CERT scores predict COPD risk, particularly among smokers. Smoking is well established as the most important risk factor for COPD, and smokers with higher CERT1 and CERT2 scores showed a greater decline in COPD-free survival, highlighting the impact of smoking on ceramide metabolism. Cigarette smoke has been shown to activate the AMPKα1/SMPD3/Cer axis, leading to ceramide accumulation, which contributes to metabolic dysfunction and steatosis [29]. These findings are consistent with our results, suggesting that elevated ceramide levels, especially in smokers, are strongly associated with COPD and underscore the role of ceramides in smoking-induced lung injury and COPD pathogenesis.
Telenga et al. (2014) conducted the only known human study demonstrating both elevated ceramide levels in smokers with COPD and a significant reduction in ceramide expression after two months of smoking cessation, reinforcing the reversible impact of smoking on ceramide metabolism [30]. Although more recent literature, including the review by Kotlyarov [6], supports the mechanistic link between smoking and ceramide dysregulation, no subsequent human studies have replicated the longitudinal findings reported by Telenga et al. Collectively, these studies highlight the contribution of ceramides to smoking-related lung injury and COPD progression in smokers. This evidence suggests that targeting ceramide metabolism may represent a promising therapeutic strategy, although further research is needed to develop clinically applicable treatments.
Ceramide-phosphatidylcholine interactions
Cer ratios, such as Cer (d18:1/18:0)/PC (14:0/22:6), were significantly associated with both prevalent and incident COPD, with slightly stronger associations observed in incident cases. This highlights the importance of the interaction between Cers and PC in understanding COPD progression. Supporting studies have shown that PC metabolism is tightly regulated in alveolar type II cells to ensure proper surfactant PC synthesis, turnover, and secretion [31]. Although saturated PC levels did not significantly change in some models, the observed ceramide–PC imbalances are consistent with prior findings suggesting that alveolar type II cell dysfunction—particularly impaired surfactant lipid synthesis and secretion—contributes to structural lung changes and COPD pathogenesis [32].
Linking COPD and cardiovascular disease (CVD)
Our findings indicate that elevated levels of specific Cer species, particularly Cer (d18:1/24:1) for prevalent COPD and the Cer (d18:1/18:0)/Cer (d18:1/24:0) ratio for incident COPD, are significantly associated with the disease. This association is likely driven by the role of Cers in promoting systemic inflammation and oxidative stress, which contribute to COPD pathogenesis. Elevated Cer levels have also been linked to vascular dysfunction, a key feature of CVD, suggesting shared pathogenic mechanisms between COPD and CVD [33]. Research over the past decade has demonstrated that both COPD and CVD share common mechanisms, including chronic low-grade systemic inflammation and endothelial dysfunction. These processes are evident even in early stages of both diseases and tend to worsen during acute exacerbations of COPD, increasing cardiovascular risk [34,35,36]. These exacerbations increase systemic inflammation, leading to the upregulation of adhesion molecules such as Macrophage-1 Antigen (MAC-1) and inflammatory mediators IL-6 and TNFα. This systemic inflammation contributes to endothelial dysfunction and increases the risk of cardiovascular complications [37,38,39]. While the lipid profiles of Cers and PCs are similar in both COPD and CVD, certain biomarkers, such as Cer (d18:1/18:0), play distinct roles in the pathogenesis of each condition. Therefore, careful interpretation of these lipid profiles is crucial in clinical settings to differentiate between COPD and CVD, ensuring accurate diagnosis and appropriate treatment strategies. Furthermore, the roles of Cers in systemic inflammation extend beyond COPD and CVD. Recent findings from our work in rheumatoid arthritis (RA) have demonstrated significant associations between CERT scores and inflammation [40], emphasizing the broader relevance of Cers in chronic inflammatory diseases. Similarly, our hypertension studies have identified specific Cer ratios, such as Cer(d18:1/18:0), as significant predictors of the onset of hypertension, reinforcing their role in linking COPD with other non-communicable diseases [41].
Although the diagnosis of COPD in this study adheres to the Finnish national guidelines, as described in the Methods [14], it also aligns with globally accepted standards, such as those outlined in the GOLD 2024 report, which provides evidence-based strategies for the prevention, diagnosis, and management of COPD [42]. It is important to acknowledge the potential variability in the implementation of these guidelines across different healthcare settings. In real-world practice, adherence to clinical guidelines may vary, which could introduce inconsistencies. However, the ascertainment of both prevalent and incident COPD cases in our study was based on in-hospital diagnoses, making these diagnoses highly reliable.
It is probable that among people classified as not having COPD, they may have mild forms of the disease, leading to false negatives. Such false negative cases could weaken or reduce the observed association. However, given that our study included incident cases of COPD over a long follow-up period, the impact of false negatives was minimized to some extent. In addition, the large size of our cohort means that any false negatives among people classified as non-COPD are unlikely to introduce significant bias.
The strength of our study lies in its focus on a cohort of middle-aged individuals, who are at the highest risk of developing COPD. This, however, limits the generalizability of our findings to elderly populations, who may exhibit different Cer profiles or COPD progression. Although we accounted for several confounding factors, there may be residual confounding from unmeasured variables. Additionally, the observational design of the study prevents us from establishing causal relationships between CERT scores and Cer ratios such as Cer (d18:1/18:0) with the COPD outcome. It remains unclear whether systemic inflammation drives Cer release or if elevated Cer levels contribute to inflammation. This underscores the need for longitudinal studies to understand this bidirectional relationship. While Cer (d18:1/18:0) and other Cer/PC ratios were significantly associated with COPD, the precise biological mechanisms underlying these associations remain unclear and warrant further investigations. Although the smoking status was included in our analysis, we could not assess the lifetime cumulative smoking exposure, and smoking habits could have been underreported, potentially influencing the associations observed between Cers and PC with the COPD outcome. In addition, we lacked data on other potential lifestyle and environmental exposures that could contribute to the risk of COPD.
Additionally, individual-level spirometry data such as Forced Expiratory Volume in 1 second (FEV₁) and the FEV₁/FVC ratio were not available in our dataset. As a result, lung function measures could not be included in the baseline characteristics table, which limits the clinical context and precludes a more detailed characterization of the cohort’s pulmonary function at baseline.
Furthermore, we did not have GOLD staging information or other severity-related data to assess or stratify participants by COPD severity. This limitation restricts our ability to adjust analyses for disease stage or to explore whether biomarker associations differ across severity levels. Nevertheless, COPD diagnoses were based on registry-confirmed clinical records in accordance with national and international guidelines that require spirometry, providing confidence in the diagnostic validity.
Furthermore, there is a chance that the regression models will overfit due to the small number of COPD patients, particularly in the prevalence study. The small number of cases may still have an impact on model stability and the generalizability of our findings, even though we reduced the number of covariates and concentrated on clinically justifiable variables to lower this risk.