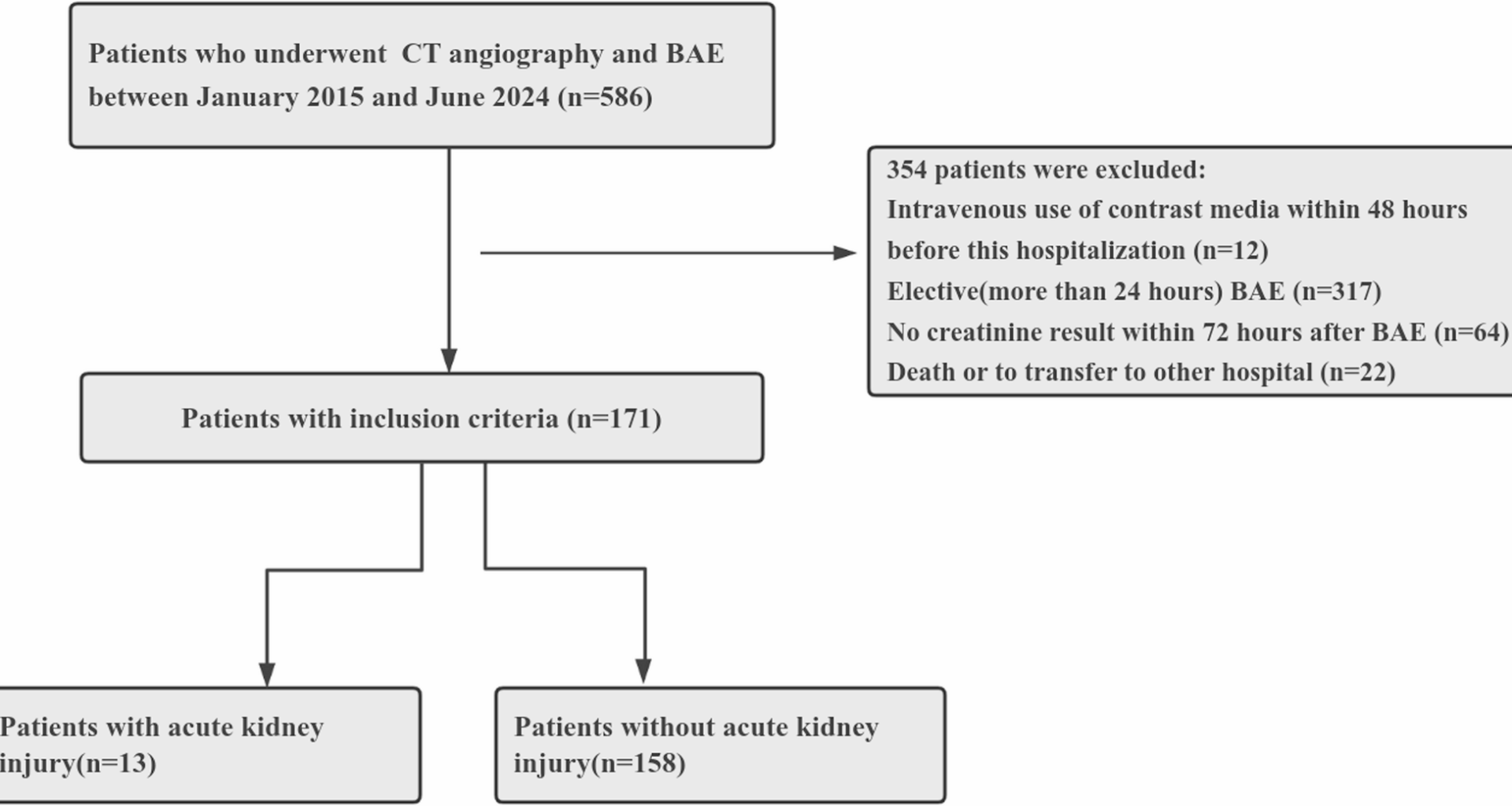
In this retrospective study, we found that the incidence of CA-AKI was 7.6%(13/171) in patients with hemoptysis treated with concomitant CTA and BAE within a short time (24 h). In addition, a proportion of those patients were known to have risk factors for CA-AKI, including age over 75 years of age, diabetes mellitus, chronic kidney disease, and glomerular filtration rate < 30 mL/min/1.73 m2.
Previous studies have demonstrated that the incidence of CA-AKI in patients with acute ischemic stroke who have undergone endovascular thrombectomy ranges from 0% to 8.7% [7,8,9]. Moreover, some studies have shown an increased rate of CA-AKI in 12–26% of patients with myocardial infarction who underwent percutaneous coronary intervention [6, 10, 15,16,17]. This complication can not only prolong the hospital stay and increase medical expenses but also induce irreversible kidney damage and increase the mortality rate of patients. The incidence of CA-AKI in our study is similar to that reported in previous studies of ischemic stroke patients who underwent concomitant CTA and cerebral angiography [7,8,9]. However, this number is low compared with that of patients who underwent percutaneous coronary intervention [6, 10, 16, 17]. The possible reasons for this difference are as follows: (1) On coronary angiography, renal artery stenosis is detected in approximately 15%−28% of patients with suspected cardiovascular disease. Renal artery stenosis exceeding 50% diminishes renal perfusion pressure, whereas stenosis over 70% decreases renal blood flow, leading to reduced glomerular filtration pressure and subsequent ischemic damage to the renal parenchyma. (2) Patients with acute coronary syndrome may also have low cardiac output, which may result in insufficient blood flow to hypoperfusion areas in the kidneys. In addition, contrast medium administered intravenously had a lower CA-AKI rate than did contrast medium administered intraarterially. First, this may be because undiluted contrast agent can reach the kidneys directly via the arterial route and cause nephrotoxic effects [11]. Second, it may also result from hypotension or the detachment of atheromatous plaque, leading to renal ischemia during the contrast procedure.
In the multivariable analysis, we found that older patients, especially those older than 75 years, were more Likely to have contrast renal injury. This is probably because serum creatinine concentrations increase with increasing age, especially after 60 years, and creatinine clearance also decreases with age [18]. In Hipp et al.‘s study, age > 75 years and preexisting renal insufficiency (SCR > 1.5 mg/dl or GFR < 60 ml/min) were reported as directly related risk factors for AKI in traumatic patients [19]. A single-center study of 292 patients from Japan revealed that age > 75 years is a risk factor for CA-AKI. CA-AKI in elderly individuals may be associated with prolonged renal dysfunction and poor outcomes [20]. Additionally, we reported that patients with diabetes mellitus, chronic kidney disease and a glomerular filtration rate < 30 mL/min/1.73 m2 are more prone to CA-AKI. These findings are consistent with those of previous investigations [6,7,8, 10]. Stolker Joshua M et al. have shown that preprocedural glucose elevations are associated with a greater risk of C-AKI in patients without known diabetes who are undergoing coronary angiography [21]. A possible reason is that those patients have reduced numbers of functioning nephrons and impaired abilities to regenerate tubular epithelium cells from iodinated contrast. Moreover, the high glucose status of diabetic patients may lead to an increase in reactive oxygen species (ROS) during oxidative damage to the kidney parenchyma (particularly outer medullary hypoxia), which leads to renal dysfunction.
In this study, patients with CA-AKI did not undergo hemodialysis or continuous renal replacement therapy and recovered their renal function within several days. Therefore, even if CA-AKI occurs, its severity is not severe. These results are consistent with those of a previous study [7]. One prospective observational study reported that the incidence of contrast-induced nephropathy was 3.3% (11/336) in endovascular thrombectomy patients. However, none of those patients required renal replacement therapy [7]. In trauma patients following contrast-enhanced imaging, McGillicuddy et al. [22] reported that only 0.3% (2/822) of patients with CA-AKI developed irreversible renal insufficiency and required hemodialysis. Thus, treatment decisions, including CTA and emergent BAE, must be based on respiratory and circulatory status, and active measures should be taken in a timely manner. Contrast-induced nephropathy is a significant contributor to hospital-acquired renal injury. The following reasons might be responsible for the underlying mechanisms of psychopathology: The pathophysiological mechanisms of CA-AKI are multifactorial, involving direct cytotoxic effects on renal tubules, vasoconstriction leading to medullary hypoxia, and increased oxidative stress [13, 15]. In our cohort, the dominant role of baseline patient factors over contrast volume in predicting CA-AKI suggests that pre-existing renal vulnerability is a critical prerequisite for these mechanisms to manifest clinically after contrast exposure. Several studies have shown a correlation between baseline renal impairment (eGFR < 30 mL/min/1.73 m2) and CA-AKI, which in turn is associated with an increased risk of mortality [7, 9, 10, 14]. It is imperative to identify high-risk groups early, select appropriate contrast agents and doses, avoid or reduce the use of nephrotoxic drugs, strengthen follow-up among high-risk groups, and identify AKI early. However, there is no current randomized trial with statistical power to provide concrete evidence of the benefits of prophylactic actions, including the use of hydration, vasodilators or antioxidant therapy, to prevent CA-AKI.
The present study has several limitations. (1) The study is a retrospective observational study involving only patients who underwent BAE at a single center, and the sample size is limited. More patients from multiple centers are needed for statistical validation. (2) There is an additional bias due to the lack of laboratory data on creatinine levels because critically ill patients treated with BAE still died and were discharged or transferred for other reasons. This may have led to an underestimation of the true incidence of CA-AKI. (3) However, our study revealed that patients with a glomerular filtration rate < 30 mL/min/1.73 m2 had a greater incidence of CA-AKI than those with other eGFR values. We did not explore the cutoff value of the baseline estimated glomerular filtration rate at which CA-AKI occurred. (4) Whether CA-AKI incidence differs between CTA and BAE or both was not further examined.
In summary, this study has revealed that those aged over 75 years, with diabetes mellitus, chronic kidney disease, and severe baseline renal impairment (eGFR < 30 mL/[min·1.73 m2]) are at an increased risk of CA-AKI, which was not associated with fatal adverse effects.While the total contrast volume within the range used was not an independent risk factor in our cohort. This shifts the focus of risk stratification from the procedure itself to the patient’s inherent vulnerability. Consequently, our work adds a new layer of evidence not on the indication for CTA, but on the safety of the integrated CTA-BAE pathway. It helps define the parameters for which this approach is safest and provides reassurance that it should not be withheld due to concerns over renal function alone.