Dutta P, Kumari A, Mahanta M, Upamanya Gunadhya K, Heisnam P et al. Nanotechnological approaches for management of soil-borne plant pathogens. 2023;14. https://doi.org/10.3389/fpls.2023.1136233.
Jang Y, Yi H, Maharjan R, Jeong M, Yoon Y. First report of root rot caused by Fusarium armeniacum on soybean in Korea. Plant Dis. 2022;106(4):1306. https://doi.org/10.1094/pdis-11-20-2319-pdn.
Cao Y, Ma J, Han S, Hou M, Wei X, et al. Single-cell RNA sequencing profiles reveal cell type-specific transcriptional regulation networks conditioning fungal invasion in maize roots. Plant Biotechnol J. 2023;21(9):1839–59. https://doi.org/10.1111/pbi.14097.
Li Z, Bai X, Jiao S, Li Y, Li P, et al. A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance. Microbiome. 2021;9(1):217. https://doi.org/10.1186/s40168-021-01169-9.
Zuriegat Q, Zheng Y, Liu H, Wang Z, Yun Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol Plant Pathol. 2021;22(7):882–95. https://doi.org/10.1111/mpp.13068.
Zhou X, Wang JT, Wang WH, Tsui CK, Cai L. Changes in bacterial and fungal microbiomes associated with tomatoes of healthy and infected by Fusarium oxysporum f. sp. lycopersici. Micro Ecol. 2021;81(4):1004–17. https://doi.org/10.1007/s00248-020-01535-4.
Preece C, Peñuelas J. A return to the wild: root exudates and food security. Trends Plant Sci. 2020;25(1):14–21. https://doi.org/10.1016/j.tplants.2019.09.010.
Jiang G, Zhang Y, Gan G, Li W, Wan W, et al. Exploring rhizo-microbiome transplants as a tool for protective plant-microbiome manipulation. ISME Commun. 2022;2(1):10. https://doi.org/10.1038/s43705-022-00094-8.
Choi K, Choi J, Lee PA, Roy N, Khan R, et al. Alteration of bacterial wilt resistance in Tomato plant by microbiota transplant. Front Plant Sci. 2020;11:1186. https://doi.org/10.3389/fpls.2020.01186.
Peterson RKD, Varella AC, Higley LG. Tolerance: the forgotten child of plant resistance. PeerJ. 2017;5: e3934. https://doi.org/10.7717/peerj.3934.
Maulenbay A, Rsaliyev A. Fungal disease tolerance with a focus on wheat: a Review. 2024;10(7):482.
Dutta P, Kumari A, Mahanta M, Upamanya Gunadhya K, Heisnam P et al. Nanotechnological approaches for management of soil-borne plant pathogens. 2023;Volume 14 – 2023. https://doi.org/10.3389/fpls.2023.1136233.
El-Shetehy M, Moradi A, Maceroni M, Reinhardt D, Petri-Fink A, et al. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat Nanotechnol. 2021;16(3):344–53. https://doi.org/10.1038/s41565-020-00812-0.
Du J, Liu B, Zhao T, Xu X, Lin H, et al. Silica nanoparticles protect rice against biotic and abiotic stresses. J Nanobiotechnol. 2022;20(1):197. https://doi.org/10.1186/s12951-022-01420-x.
Zhao L, Lu L, Wang A, Zhang H, Huang M, et al. Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J Agric Food Chem. 2020;68(7):1935–47. https://doi.org/10.1021/acs.jafc.9b06615.
Debona D, Rodrigues FA, Datnoff LE. Silicon’s role in abiotic and biotic plant stresses. Annu Rev Phytopathol. 2017;55:85–107. https://doi.org/10.1146/annurev-phyto-080516-035312.
Deng Q, Liu H, Lu Q, Gangurde SS, Du P, et al. Silicon application for the modulation of rhizosphere soil bacterial community structures and metabolite profiles in Peanut under Ralstonia solanacearum inoculation. 2023;24(4):3268.
Deng Q, Huang S, Liu H, Lu Q, Du P et al. Silica nanoparticles conferring resistance to bacterial wilt in peanut (Arachis hypogaea L.). Sci Total Environ. 2024;915:170112. https://doi.org/10.1016/j.scitotenv.2024.170112.
Gao CH, Zhang M, Wu Y, Huang Q, Cai P. Divergent influence to a pathogen invader by resident bacteria with different social interactions. Microb Ecol. 2019;77(1):76–86. https://doi.org/10.1007/s00248-018-1207-z.
Li M, Wei Z, Wang J, Jousset A, Friman VP, et al. Facilitation promotes invasions in plant-associated microbial communities. Ecol Lett. 2019;22(1):149–58. https://doi.org/10.1111/ele.13177.
Gu S, Wei Z, Shao Z, Friman VP, Cao K, et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat Microbiol. 2020;5(8):1002–10. https://doi.org/10.1038/s41564-020-0719-8.
Li M, Pommier T, Yin Y, Wang J, Gu S, et al. Indirect reduction of Ralstonia solanacearum via pathogen helper inhibition. ISME J. 2021;16(3):868–75. https://doi.org/10.1038/s41396-021-01126-2.
Jiang T, Ren J, Li D, Luo Y, Huang Y et al. Pseudomonas syringae exacerbates apple replant disease caused by Fusarium. Microbiol Res. 2025;296:128124. https://doi.org/10.1016/j.micres.2025.128124.
Li M, Wang Q, Liu Z, Pan X, Zhang Y. Silicon application and related changes in soil bacterial community dynamics reduced ginseng black spot incidence in Panax ginseng in a short-term study. BMC Microbiol. 2019;19(1):263. https://doi.org/10.1186/s12866-019-1627-z.
Fortunato AA, da Silva WL, Rodrigues F. Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporum f. sp. cubense. Phytopathology. 2014;104(6):597–603. https://doi.org/10.1094/phyto-07-13-0203-r.
Tripathi DK, Vishwakarma K, Singh VP, Prakash V, Sharma S, et al. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J Hazard Mater. 2021;408: 124820. https://doi.org/10.1016/j.jhazmat.2020.124820.
Ye M, Song Y, Long J, Wang R, Baerson SR et al. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. 2013;110(38):E3631-E3639. https://doi.org/10.1073/pnas.1305848110.
Kurabachew H, Wydra K. Induction of systemic resistance and defense-related enzymes after elicitation of resistance by rhizobacteria and silicon application against Ralstonia solanacearum in tomato (Solanum lycopersicum). Crop Prot. 2014;57:1–7. https://doi.org/10.1016/j.cropro.2013.10.021.
Yuan J, Zhao J, Wen T, Zhao M, Li R, et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome. 2018;6(1):156. https://doi.org/10.1186/s40168-018-0537-x.
Yang M, Li Z, Liu L, Bo A, Zhang C et al. Ecological niche modeling of Astragalus membranaceus var. mongholicus medicinal plants in Inner Mongolia, China. Sci Rep. 2020;10(1):12482. https://doi.org/10.1038/s41598-020-69391-3.
Feng J, Chen Q, Wu X, Jafari SM, McClements DJ. Formulation of oil-in-water emulsions for pesticide applications: impact of surfactant type and concentration on physical stability. Environ Sci Pollut Res. 2018;25(22):21742–51. https://doi.org/10.1007/s11356-018-2183-z.
Wang P, Zhang H, Hu X, Xu L, An X et al. Comparing the potential of silicon nanoparticles and conventional silicon for salinity stress alleviation in Soybean (Glycine max L.): Growth and physiological traits and rhizosphere/endophytic bacterial communities. J Agric Food Chem. 2024;72(19):10781–93. https://doi.org/10.1021/acs.jafc.4c00154.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. https://doi.org/10.1038/nmeth.f.303.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–8. https://doi.org/10.1038/nmeth.2604.
Oppenheimer-Shaanan Y, Jakoby G, Starr ML, Karliner R, Eilon G et al. A dynamic rhizosphere interplay between tree roots and soil bacteria under drought stress. eLife. 2022;11. https://doi.org/10.7554/eLife.79679.
Sun X, Xu Z, Xie J, Hesselberg-Thomsen V, Tan T, et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022;16(3):774–87. https://doi.org/10.1038/s41396-021-01125-3.
Bergkessel M, Guthrie C. Chapter Twenty Five – Colony PCR. In: Lorsch J (editor). Methods enzymol: academic Press; 2013. pp. 299–309.
Zhou Y, Yang Z, Liu J, Li X, Wang X, et al. Crop rotation and native microbiome inoculation restore soil capacity to suppress a root disease. Nat Commun. 2023;14(1):8126. https://doi.org/10.1038/s41467-023-43926-4.
McLaughlin S, Zhalnina K, Kosina S, Northen TR, Sasse J. The core metabolome and root exudation dynamics of three phylogenetically distinct plant species. Nat Commun. 2023;14(1):1649. https://doi.org/10.1038/s41467-023-37164-x.
Zhou X, Zhang J, Khashi u Rahman M, Gao D, Wei Z et al. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol Plant. 2023;16(5):849–864. https://doi.org/10.1016/j.molp.2023.03.009.
Fu Q, Lai JL, Ji XH, Luo ZX, Wu G, et al. Alterations of the rhizosphere soil microbial community composition and metabolite profiles of Zea mays by polyethylene-particles of different molecular weights. J Hazard Mater. 2022;423(Pt A): 127062. https://doi.org/10.1016/j.jhazmat.2021.127062.
Yu G, Xu C, Zhang D, Ju F, Ni Y. MetOrigin: discriminating the origins of microbial metabolites for integrative analysis of the gut microbiome and metabolome. iMeta. 2022;1(1):e10. https://doi.org/10.1002/imt2.10.
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–21. https://doi.org/10.1038/nbt.2676.
Burns AR, Stephens WZ, Stagaman K, Wong S, Rawls JF, et al. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016;10(3):655–64. https://doi.org/10.1038/ismej.2015.142.
Ning D, Yuan M, Wu L, Zhang Y, Guo X, et al. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat Commun. 2020;11(1):4717. https://doi.org/10.1038/s41467-020-18560-z.
Rosindell J, Hubbell SP, Etienne RS. The unified neutral theory of biodiversity and biogeography at age ten. Trends Ecol Evol. 2011;26(7):340–348. https://doi.org/10.1016/j.tree.2011.03.024.
Chen W, Ren K, Isabwe A, Chen H, Liu M, et al. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome. 2019;7(1):138. https://doi.org/10.1186/s40168-019-0749-8.
Wang M, Gao L, Dong S, Sun Y, Shen Q et al. Role of silicon on plant–pathogen interactions. 2017;8. https://doi.org/10.3389/fpls.2017.00701.
Ahammed GJ, Yang Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol Biochem : PPB. 2021;165:200–6. https://doi.org/10.1016/j.plaphy.2021.05.031.
Zhou T, Guo T, Wang Y, Wang A, Zhang M. Carbendazim: ecological risks, toxicities, degradation pathways and potential risks to human health. Chemosphere. 2023;314:137723. https://doi.org/10.1016/j.chemosphere.2022.137723.
Kumari K, Rani N, Hooda V. Unravelling the effects of nano SiO2, nano TiO2 and their nanocomposites on Zea mays L. growth and soil health. Sci Rep. 2024;14(1):13996. https://doi.org/10.1038/s41598-024-61456-x.
Mostofa SM. Introduction. In: Mostofa SM, editor. Islamist militancy in Bangladesh: a pyramid root cause model. Cham: Springer International Publishing; 2021. p. 1–36.
Tian L, Shen J, Sun G, Wang B, Ji R et al. Foliar Application of SiO(2) nanoparticles alters soil metabolite profiles and microbial community composition in the Pakchoi (Brassica chinensis L.) rhizosphere grown in contaminated mine soil. Environ Sci Technol. 2020;54(20):13137–46. https://doi.org/10.1021/acs.est.0c03767.
Schwab F, Zhai G, Kern M, Turner A, Schnoor JL, et al. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–Critical review. Nanotoxicology. 2016;10(3):257–78. https://doi.org/10.3109/17435390.2015.1048326.
Garbeva P, van Veen JA, van Elsas JD. Microbial diversity in soil: selection microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol. 2004;42:243–70. https://doi.org/10.1146/annurev.phyto.42.012604.135455.
Kwak MJ, Kong HG, Choi K, Kwon SK, Song JY, et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat Biotechnol. 2018. https://doi.org/10.1038/nbt.4232.
Spragge F, Bakkeren E, Jahn MT, B. N. Araujo E, Pearson CF et al. Microbiome diversity protects against pathogens by nutrient blocking. Science (New York, NY).382(6676):eadj3502. https://doi.org/10.1126/science.adj3502.
Fang S, Liu D, Tian Y, Deng S, Shang X. Tree species composition influences enzyme activities and microbial biomass in the rhizosphere: a rhizobox approach. PLoS ONE. 2013;8(4): e61461. https://doi.org/10.1371/journal.pone.0061461.
Liu K, Cai M, Hu C, Sun X, Cheng Q et al. Selenium (Se) reduces Sclerotinia stem rot disease incidence of oilseed rape by increasing plant Se concentration and shifting soil microbial community and functional profiles. Environ Pollut. 2019;254:113051. https://doi.org/10.1016/j.envpol.2019.113051.
Bi Y, Tian SP, Guo YR, Ge YH, Qin GZ. Sodium silicate reduces postharvest decay on hami melons: induced resistance and fungistatic effects. Plant Dis. 2006;90(3):279–83. https://doi.org/10.1094/pd-90-0279.
Zhou X, Shen Y, Fu X, Wu F. Application of sodium silicate enhances cucumber resistance to Fusarium wilt and alters soil microbial communities. Front Plant Sci. 2018;9:624. https://doi.org/10.3389/fpls.2018.00624.
Ma C, Ci K, Zhu J, Sun Z, Liu Z, et al. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. The Science of the total environment. 2021;759: 143501. https://doi.org/10.1016/j.scitotenv.2020.143501.
Deng Q, Yu T, Zeng Z, Ashraf U, Shi Q, et al. Silicon application modulates the growth, rhizosphere soil characteristics, and bacterial community structure in sugarcane. Front Plant Sci. 2021;12: 710139. https://doi.org/10.3389/fpls.2021.710139.
Lin L, Shao X, Yang Y, Murero AK, Wang L et al. Lysobacter enzymogenes: a fully armed biocontrol warrior1. J Integr Agric. 2024. https://doi.org/10.1016/j.jia.2024.02.021.
Wang B, Xia Q, Lin Y, Wei F, Yang S et al. Root rot induces a core assemblage of bacterial microbiome to prevent disease infection in Sanqi ginseng. Appl Soil Ecol. 2024;198:105371. https://doi.org/10.1016/j.apsoil.2024.105371.
Walker TS, Bais HP, Déziel E, Schweizer HP, Rahme LG et al. Pseudomonas aeruginosa-plant root interactions. Pathogenicity, Biofilm Formation, and Root Exudation. Plant Physiol. 2004;134(1):320–31. https://doi.org/10.1104/pp.103.027888.
Solis-Ortiz CS, Gonzalez-Bernal J, Kido-Díaz HA, Peña-Uribe CA, López-Bucio JS, et al. Bacterial cyclodipeptides elicit Arabidopsis thaliana immune responses reducing the pathogenic effects of Pseudomonas aeruginosa PAO1 strains on plant development. J Plant Physiol. 2022;275: 153738. https://doi.org/10.1016/j.jplph.2022.153738.
Starkey M, Rahme LG. Modeling Pseudomonas aeruginosa pathogenesis in plant hosts. Nat Protoc. 2009;4(2):117–24. https://doi.org/10.1038/nprot.2008.224.
Yuan QS, Gao Y, Wang L, Wang X, Wang L, et al. Pathogen-driven Pseudomonas reshaped the phyllosphere microbiome in combination with Pseudostellaria heterophylla foliar disease resistance via the release of volatile organic compounds. Environ Microbiome. 2024;19(1):61. https://doi.org/10.1186/s40793-024-00603-3.
Rudrappa T, Splaine RE, Biedrzycki ML, Bais HP. Cyanogenic pseudomonads influence multitrophic interactions in the rhizosphere. PLoS ONE. 2008;3(4): e2073. https://doi.org/10.1371/journal.pone.0002073.
Vacheron J, Péchy-Tarr M, Brochet S, Heiman CM, Stojiljkovic M, et al. T6SS contributes to gut microbiome invasion and killing of an herbivorous pest insect by plant-beneficial Pseudomonas protegens. ISME J. 2019;13(5):1318–29. https://doi.org/10.1038/s41396-019-0353-8.
Gallegos-Monterrosa R, Coulthurst SJ. The ecological impact of a bacterial weapon: microbial interactions and the Type VI secretion system. FEMS Microbiol Rev. 2021;45(6):fuab033. https://doi.org/10.1093/femsre/fuab033.
Liu X, Matsumoto H, Lv T, Zhan C, Fang H, et al. Phyllosphere microbiome induces host metabolic defence against rice false-smut disease. Nat Microbiol. 2023;8(8):1419–33. https://doi.org/10.1038/s41564-023-01379-x.
Li Q, Liu Z, Jiang Z, Jia M, Hou Z et al. Phenylalanine metabolism-dependent lignification confers rhizobacterium-induced plant resistance. Plant Physiol. 2025;197(2). https://doi.org/10.1093/plphys/kiaf016.
Stegen JC, Lin X, Fredrickson JK, Chen X, Kennedy DW, et al. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013;7(11):2069–79. https://doi.org/10.1038/ismej.2013.93.
Jiang Y, Wang W, Xie Q, Liu N, Liu L et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. 2017;356(6343):1172–1175. https://doi.org/10.1126/science.aam9970.
Verma V, Ravindran P, Kumar PP. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016;16:86. https://doi.org/10.1186/s12870-016-0771-y.
Cha J-Y, Han S, Hong H-J, Cho H, Kim D, et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016;10(1):119–29. https://doi.org/10.1038/ismej.2015.95.
Morgunov IG, Kamzolova SV, Dedyukhina EG, Chistyakova TI, Lunina JN, et al. Application of organic acids for plant protection against phytopathogens. Appl Microbiol Biotechnol. 2017;101(3):921–32. https://doi.org/10.1007/s00253-016-8067-6.
Ahammed GJ, Li X, Liu A, Chen S. Physiological and defense responses of Tea plants to elevated CO2: a review. 2020;11. https://doi.org/10.3389/fpls.2020.00305.
Sun M, Li L, Wang C, Wang L, Lu D, et al. Naringenin confers defence against Phytophthora nicotianae through antimicrobial activity and induction of pathogen resistance in tobacco. Mol Plant Pathol. 2022;23(12):1737–50. https://doi.org/10.1111/mpp.13255.
Yang K, Zhou G, Chen C, Liu X, Wei L et al. Joint metabolomic and transcriptomic analysis identify unique phenolic acid and flavonoid compounds associated with resistance to fusarium wilt in cucumber (Cucumis sativus L.). Frontiers in plant science. 2024;15:1447860. https://doi.org/10.3389/fpls.2024.1447860.
Zhang Y, Chen G, Zang Y, Bhavani S, Bai B et al. Lr34/Yr18/Sr57/Pm38 confers broad-spectrum resistance to fungal diseases via transport of sinapyl alcohol for cell wall lignification in wheat. Plant Commun. 2024:101077. https://doi.org/10.1016/j.xplc.2024.101077.
Dos Santos C, Franco OL. Pathogenesis-related proteins (PRs) with enzyme activity activating plant defense responses. Plants (Basel, Switzerland). 2023;12(11). https://doi.org/10.3390/plants12112226.
An C, Mou Z. Salicylic acid and its function in plant immunity. J Integr Plant Biol. 2011;53(6):412–28. https://doi.org/10.1111/j.1744-7909.2011.01043.x.
Zeng H, He K, He Q, Xu L, Zhang W, et al. Exogenous indole-3-acetic acid suppresses rice infection of Magnaporthe oryzae by affecting plant resistance and fungal growth. Phytopathology. 2024;114(5):1050–6. https://doi.org/10.1094/phyto-10-23-0365-kc.
Xia Y, Yu K, Navarre D, Seebold K, Kachroo A, et al. The glabra1 mutation affects cuticle formation and plant responses to microbes. Plant Physiol. 2010;154(2):833–46. https://doi.org/10.1104/pp.110.161646
.
Bano K, Kumar B, Alyemeni MN, Ahmad P. Exogenously-sourced salicylic acid imparts resilience towards arsenic stress by modulating photosynthesis, antioxidant potential and arsenic sequestration in Brassica napus plants. Antioxidants (Basel, Switzerland). 2022;11(10). https://doi.org/10.3390/antiox11102010
.
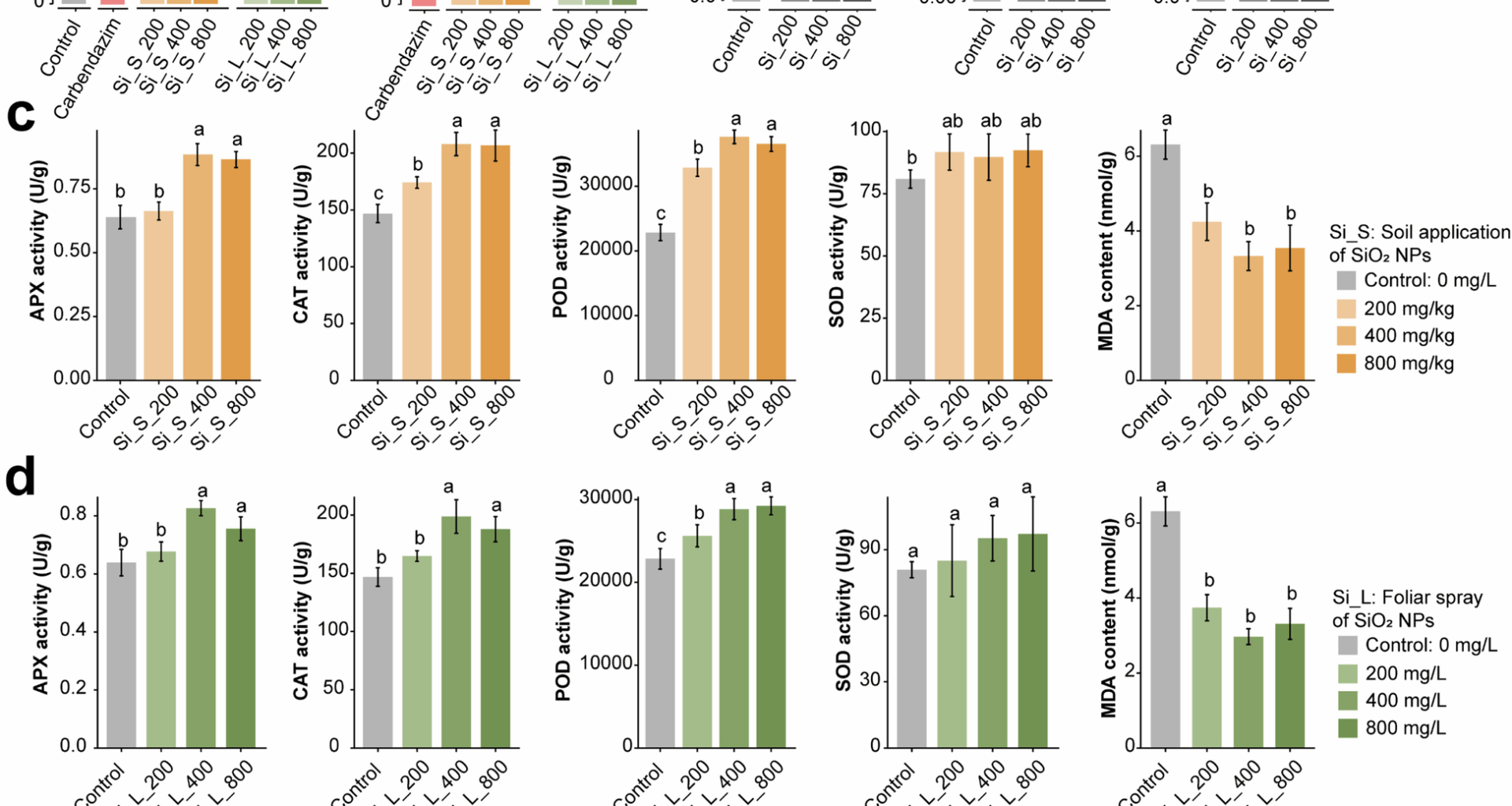
Sun S, Yang Z, Song Z, Wang N, Guo N et al. Silicon enhances plant resistance to Fusarium wilt by promoting antioxidant potential and photosynthetic capacity in cucumber (Cucumis sativus L.). Front Plant Sci. 2022;13:1011859. https://doi.org/10.3389/fpls.2022.1011859.
Carneiro-Carvalho A, Pinto T, Ferreira H, Martins L, Pereira C et al. Effect of silicon fertilization on the tolerance of Castanea sativa Mill. seedlings against Cryphonectria parasitica Barr. J Plant Dis Prot. 2020;127(2):197–210. https://doi.org/10.1007/s41348-019-00283-z.