Chiroptical properties of discrete (S/R)-Ag6 clusters (stage I)
The construction of atomically precise metal clusters with CPL by direct or post-modification synthesis of peripheral chiral ligands is a convenient and efficient approach39,40,41,42. On the basis of this strategy, an enantiomeric pair of Ag(I) clusters capped by the chiral ligand (S/R)−4-isopropylthiazolidine-2-thione was constructed in our previous work19; this pair has excellent solvent stability and thermal stability (Fig. 2A and Supplementary Fig. 1 and Supplementary Figs. 5–8). The geometric configuration of S/R-Ag6 is shown in Fig. 2A, where the Ag6 core is a twisted octahedron. The molecular structure of S-Ag6 was confirmed by electrospray ionisation mass spectrometry (ESI-MS) (Fig. 2B) and powder X-ray diffraction (PXRD) (Fig. 2D). The ultraviolet‒visible (UV‒vis) absorption and emission spectra of S-Ag6 in dichloromethane (DCM) are shown in Fig. 2C. Three absorption peaks centred at 392, 306, and 238 nm and an emission peak centred at 576 nm were observed. The circular dichroism (CD) and CPL spectra of (S/R)-Ag6 in DCM (10−4 mol·L−1) were determined. The origin of chirality in (S/R)-Ag6 metal clusters is extrinsic chirality induced by peripheral homochiral ligands. As shown in Fig. 2E–G, the (S/R)-Ag6 enantiomers showed obvious mirror Cotton effects and CPL responses with rather weak glum factors (± 1 × 10−3), and these glum factors were far from meeting the practical requirements. Therefore, adopting appropriate strategies to increase the glum factor is urgently needed.
Fig. 2: Structure and optical analysis of enantiomeric silver clusters (stage I).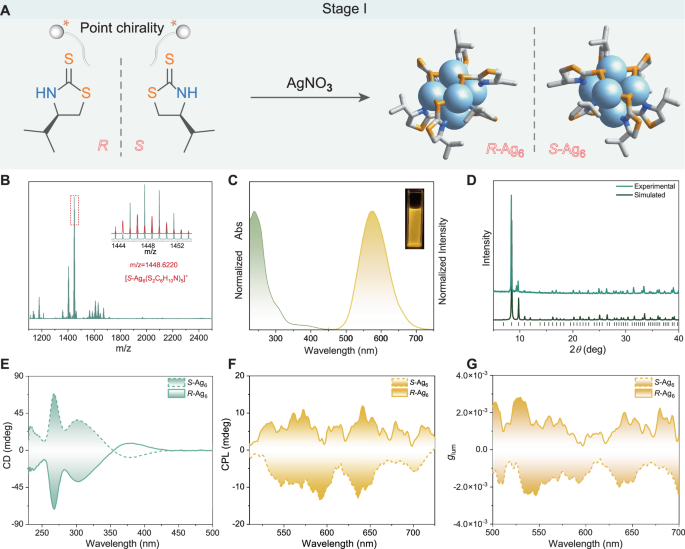
A Synthetic illustration of the desired Ag6 clusters. Colour codes: S, orange; Ag, blue; N, blue; and C, grey. H atoms are omitted for clarity. B Positive mode ESI‒MS spectra of S-Ag6 dissolved in DMF. Insets: Enlarged portion of the ESI‒MS exhibiting the measured (green line) and simulated (red line) isotopic distribution patterns in the m/z range of 1100–2500 with a charge state of + 1. The dominant peak for S-Ag6 can be assigned to [S-Ag6(S2C6H10N)5]+ (m/z = 1448.6220). C Normalised absorption and emission spectra of S-Ag6 (λex = 370 nm) in DCM solution. (Inset: image of S-Ag6 under UV light in DCM at room temperature). D PXRD patterns of simulated and as-prepared crystals of S-Ag6. E CD spectra of (S/R)-Ag6 in DCM solution. F CPL spectra of (S/R)-Ag6 in DCM solution (λex = 370 nm). G glum curves of (S/R)-Ag6 in DCM solution.
Chiroptical properties of the LC assemblies (stage II and stage III)
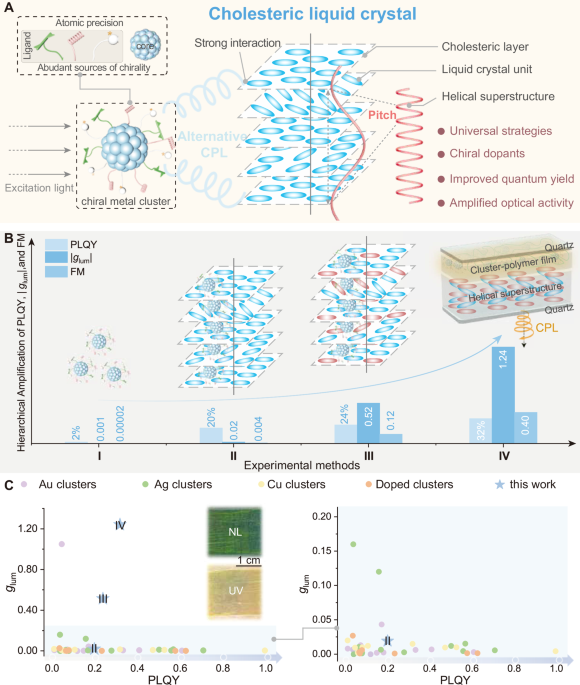
CLCs are known as excellent CPL support matrices since their unique chiral superstructure and helical arrangement form a ‘standing helix’ (SH) orientation in a planar cell43, and regulation of the helical pitch length allows for tuneable, selective reflection of circularly polarised light, realising CPL-active CLCs44. Presently, most CLCs are synthesised by doping chiral dopants with LC-like shapes into nematic LCs, and whether spherical-like clusters as chiral dopants can form CLCs is still unknown. The intrinsic chirality of (S/R)-Ag6 motivated us to further exploit its potential. As shown in Fig. 3A, the achiral room-temperature nematic LC host (SLC1717) was doped with (S/R)-Ag6 via codissolution (stage II). Then, we filled the available assemblies (S-Ag6/SLC1717 and R-Ag6/SLC1717) into cells with a cell gap of approximately 40 µm at a temperature above their clearing points. Compared with the discrete state, the emission wavelength of R-Ag6 in SLC1717 hardly changed (Fig. 3B and Supplementary Fig. 9). Upon doping, the nematic liquid crystal host (SLC1717) with Ag6 clusters, the full width of half-maxima (FWHM) for the emission spectra of Ag6 clusters decreased sharply, which could be attributed to the suppression of cluster molecular vibrations by the viscosity of the SLC1717, imparting rigidity to the luminescent metal clusters. PXRD revealed that R-Ag6/SLC1717 had diffraction patterns similar to those of the original SLC1717, indicating that R-Ag6 could be well dispersed in the LC matrix without destroying the texture45 (Supplementary Fig. 10). Increasing the mass ratio of chiral dopants within a certain range is conducive to the formation of CLCs10. However, when the weight ratio of (S/R)-Ag6/SLC1717 exceeded 1 wt%, an obvious phase separation phenomenon occurred (Supplementary Fig. 11). Considering the AIE characteristics of the enantiomeric Ag6 clusters, we used (S/R)-Ag6 as a chiral dopant (maximum of 1 wt%) to fabricate LC assemblies (S-Ag6/SLC1717 and R-Ag6/SLC1717) for subsequent research. The exciting results prompted us to thoroughly explore the chiroptical activities of (S/R)-Ag6 in LCs. Hardly detectable CD signals were observed for SLC1717, and weak CD signals were observed for (S/R)-Ag6 (Supplementary Fig. 12), whereas intense CD signals were observed in the CLC assemblies (Fig. 3C), verifying the role of (S/R)-Ag6 in chirality formation and amplification. In addition, Fig. 3D showed the CPL spectrum of (S/R)-Ag6, their |glum| factor of (S/R)-Ag6 after assembly with SLC1717 significantly increased to 0.02 (Supplementary Fig. 13), which was 20 times greater than that of the discrete state (DCM, 6×10-4 M). Notably, compared with (S/R)-Ag6, the CPL activities of (S/R)-Ag6 in the LC assemblies were inverted, which indicated that control of chirality was accomplished by high-level helical assemblies beyond the chirality of discrete enantiomeric clusters. According to the signs of the CPL, R-Ag6 and S-Ag6 clearly induced SLC1717 to become a right-handed or left-handed chiral nematic LC, respectively. Furthermore, owing to the poor compatibility of (S/R)-Ag6 with SLC1717, selective reflection was not observed (Supplementary Fig. 14) which indicated that the helical arrangement of the (S/R)-Ag6/SLC1717 assemblies was not good, resulting in a low helical twisting power (HTP) of (S/R)-Ag646, and the photonic bandgap (PBG) of the assemblies could not be regulated in the visible light region (Supplementary Figs. 15 and 16)47,48. To confirm the integrity of the silver clusters, the emission and excitation spectra of the R-Ag6 were recorded in the presence of the chiral dopants S811 and SLC1717 (Supplementary Fig. 17). However, enantiomeric clusters could be new candidates for chiral dopants if the compatibility between metal clusters and LCs is improved, and relevant research is currently underway. Although the glum factors of (S/R)-Ag6 in SLC1717 were amplified in stage II, there is still significant room for improvement, indicating that a stronger HTP effect is needed to form helical superstructures.
Fig. 3: Chiroptical properties of (S/R)-Ag6 in stage II.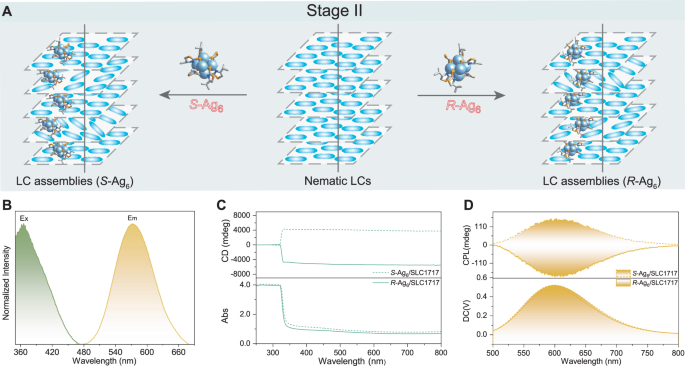
A Schematic illustration of the formation of the LC assemblies. B Normalised excitation and emission spectra of R-Ag6-based LC assemblies. C CD spectra of the LC assemblies showing mirror-image signals. The weight ratio of (R/S)-Ag6/SLC1717 was 1 wt%. D CPL spectra of the LC assemblies. The weight ratio of (R/S)-Ag6/SLC1717 was 1 wt%, and the excitation wavelength was 370 nm.
For this purpose, we selected an enantiomeric pair of chiral dopants (R811/S811) with high HTP to well twist the LC host SLC1717 (Supplementary Fig. 18) and thereby enhance the helicity of the upper helical superstructure to further increase the glum factor of the metal clusters by tuning the PBG. First, the reflection spectra across the visible light region were obtained by accurately tuning the weight ratios of R811/S811 and SLC1717 from 0.2 to 0.32 to study the PBG effect, and the natural light reflection colours simultaneously varied from dark red to green (Fig. 4A). Notably, the chiral signals of SLC1717 and R/S811 were separately examined (Supplementary Fig. 19), in which R811 and S811 presented weak CD signals ranging from 200 nm and 350 nm, whereas SLC1717 was CD silent. More interestingly, due to the helicity of all the CLCs with different doping amounts, intense mirror-image CD intensities (ca. 30000 mdeg) were distinctly observed (Fig. 4B), which validated the role of the designed helical-coassembly strategy in chirality formation and adjustment and amplification of the upper helical superstructure via the molecular chirality. Unlike the LC texture of SLC1717, oily streak textures could be clearly observed for all the CLC systems composed of R811/S811 and SLC171746,49; more specifically, these textures were regarded as being characteristic of CLC phases with a helix-packing structure in which the helical axis was perpendicular to the substrate (Supplementary Fig. 20), indicating that in the coassembly system, R811/S811 and SLC1717 were assembled into CLCs with helical packing superstructures to realise chiral transfer and amplification effects, and the driving force for superstructure formation could be attributed to the interactions between R811/S811 and SLC171750. When the chiral dopants with different weight ratios were blended with the systems in stage II, new ternary-component G-CLC assemblies (metal clusters/chiral dopants/LCs) were formed (stage III), as shown in Fig. 4C. Considering the expected chirality of the CLCs and the luminescent properties of the metal clusters, the chiroptical activities of the ternary system were investigated next. In detail, the mirror-image CD intensities of the ternary-component G-CLC assemblies were more or less weakened, implying that the introduction and even aggregation of silver clusters deformed the helical arrangement of the LC units in the doped G-CLC assemblies, leading to defects in the helical structural arrangement and further resulting in degradation of the circular polarisation performance (Fig. 4D–F and Supplementary Figs. 21 and 22). The excitation and emission spectra of G-CLC are shown in Supplementary Fig. 23, confirming the structural integrity of the silver clusters within the system. The reflected colour of the G-CLC ternary assemblies originated from the Bragg reflection and CD characteristics resulting from the periodic helical superstructure (Supplementary Figs. 24 and 25).
Fig. 4: Chiroptical properties of (S/R)-Ag6 in stage III.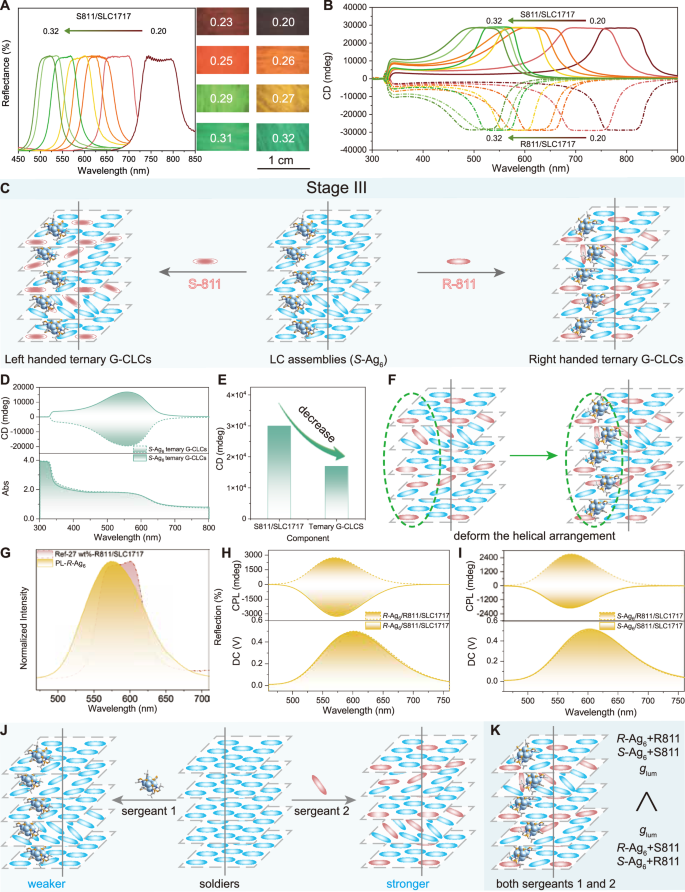
A Reflection spectra of R811/SLC1717 at different weight ratios from 20 wt% to 32 wt%. Inset: Corresponding images under NL. B CD spectra of S811/SLC1717 and R811/SLC1717 at different weight ratios from 20 wt% to 32 wt%. C Schematic illustration of the formation of the ternary G-CLCs. D CD spectra of the ternary-component G-CLCs. E Change in the CD intensity from S811/SLC1717 to the ternary-component CLC assemblies. F Schematic illustration of the deformation of the helical arrangement after the introduction of (S/R)-Ag6. G Overlap of the reflection spectra and PL spectra of R-Ag6. H CPL spectra of the ternary G-CLCs (R-Ag6/R811/SLC1717 and R-Ag6/S811/SLC1717); the excitation wavelength is 370 nm. I CPL spectra of the ternary G-CLCs (S-Ag6/R811/SLC1717 and S-Ag6/S811/SLC1717); the excitation wavelength is 370 nm. J Schematic illustration of the chiral amplification principle in the systems, i.e., the sergeants-and-soldiers rule. Sergeant 1, (S/R)-Ag6; sergeant 2, R811/S811; solider, nematic LC. K Illustration of an enantioselective effect in the ternary assemblies (both sergeants 1 and 2).
The CPL properties of the ternary assemblies were subsequently characterised by a spectrometer (JASCO CPL-300) equipped with an Xe lamp with a monochromator. Enabling the PBG of CLCs to cover the emission band of metal clusters is conducive to amplifying their CPL activities because of a circular Bragg phenomenon. For example, owing to the forbidden propagation of CPL emission with a certain handedness from CPL-active silver clusters within the PBG, these clusters can emit CPL with the opposite handedness to their helical superstructure, analogous to the purification of CPL. When the weight ratio of S811/SLC1717 was 27 wt%, unless otherwise stated, the corresponding PBG and emission band of the silver clusters overlapped (Fig. 4G), and this phenomenon was utilised for the subsequent study of the silver cluster ternary assemblies. Supplementary Fig. 26 shows that the rac-Ag6-based ternary G-CLC assemblies exhibited good mirror-image CPL signals, with the glum factor of − 0.61 at 580 nm, which was higher than that of most reported CPL-active systems (Fig. 1C)3,40,51. Notably, left-handed CPL emissions were obtained in silver cluster-doped right-handed CLCs owing to the forbidden propagation of R-CPL emissions within the PBG under the irradiation of UV light, whereas R-CPL emissions were obtained in silver cluster-doped left-handed CLCs4. Here, whether the chirality of the silver clusters can influence the CPL activities of ternary assemblies is an interesting question worthy of further exploration, which will be beneficial to the understanding of chiral amplification in supramolecular assemblies. In stage III, R811 and S811 were blended into stage II assemblies (S-Ag6/SLC1717 and R-Ag6/SLC1717), and then, enantiomeric ternary assemblies based on R-Ag6 and S-Ag6 were constructed for further study. Their glum factors were slightly lower than those of rac-Ag6-based ternary G-CLC assemblies (Supplementary Fig. 27), which indicated that the chiroptical activities of the silver clusters (guests) weakly influenced the CPL activities of the ternary assemblies. Apart from the difference in CPL activities between the chiral silver clusters and achiral silver cluster ternary assemblies, more interestingly, in the (S/R)-Ag6 ternary assemblies, the CPL activity (|glum | , 0.52) of the R-Ag6/S811/SLC1717 assemblies was superior to that (glum, 0.43) of the R-Ag6/R811/SLC1717 assemblies monitored at 574 nm, whereas the CPL activity (|glum | , 0.29) of the S-Ag6/S811/SLC1717 assemblies was weaker than that (glum, 0.43) of the S-Ag6/R811/SLC1717 assemblies monitored at 574 nm (Fig. 4H, I). The reason might be that the chirality-compatible ternary assemblies permit an enantioselective effect based on the strong interactions between the enantiomeric clusters and the CLCs. The above results demonstrated that the chirality of the silver clusters did not significantly affect the CPL activities of the ternary assemblies, further contributing to the understanding of the “sergeants-and-soldiers” principle regarding the chiral amplification effects of supramolecular assemblies52,53. Specifically, in the ternary helical superstructure assemblies, R811/S811 (sergeants) with strong HTP dominated the amplification of chirality from the molecular level to supramolecular chirality compared with the silver clusters (sergeants) with a weak HTP, even in the presence of two types of chiral sergeant molecules with opposite handedness (Fig. 4J, K). Hence, the screening, design, and construction of chiral dopant molecules are of paramount importance in the successful formation of highly helical superstructure assemblies from nematic LCs. The CPL activities of the ternary assemblies with R811/S811 and SLC1717 of various mixing weight ratios (23 wt%, 25, 29, 31, and 32 wt%) were thoroughly investigated, verifying that the highest glum factor was indeed obtained at the mixing weight ratio of 27 wt% (Supplementary Fig. 28). The results showed that similar oily streak textures were observed for the ternary G-CLC assemblies (Supplementary Fig. 29), indicating the retention of helical superstructures after doping with metal clusters.
Moreover, PXRD and differential scanning calorimetry (DSC) analyses were also performed to study the LC behaviour. All samples showed diffraction peaks similar to those of SLC1717 without a crystallisation diffraction peak, indicating that the dopants were uniformly dispersed in the LC matrix in a discrete state (Supplementary Fig. 30). Compared with those of the original SLC1717, the clearing points of the CLC assemblies decreased to various degrees (Supplementary Fig. 31). In addition, the PLQY is also an important indicator for evaluating CPL-active materials. The PLQY and lifetime of R-Ag6 in stage I (discrete state) were only 2% and 0.6 μs, respectively, whereas they increased to 20% and 1.9 μs in stage II (LC assemblies) and 24% and 2.1 μs in stage III (G-CLCs) (Supplementary Figs. 32 and 33). This behaviour could be attributed to the viscous feature of the LC matrix, which enhanced the structural rigidity of the silver clusters and inhibited nonradiative decay. Although the glum factors of the silver clusters were significantly improved by 550 times compared with those of their discrete state, there is still a gap with the ideal glum factors, making the efficient realisation of CPL practicality impossible.
CPL behaviour in silver cluster‒LC bilayer architecture devices (stage IV)
To address this major hurdle, we fabricate silver clusters-LC (SCLC) devices with a bilayer architecture to increase the glum factor. Initially, LC cells were constructed by bonding a quartz substrate coated with a polymer‒silver cluster composite film to another quartz substrate (Fig. 5A). To ensure good compatibility of the polymer and the silver cluster, we screened several polymer matrices, such as polymethyl methacrylate (PMMA), polyacrylonitrile (PAN), and polyvinylidene fluoride (PVDF). Specifically, R-Ag6 was mixed with only PMMA and dissolved in 1,2-dichloroethane to form a transparent and uniform solution (Supplementary Fig. 34), which was then spun on a quartz substrate to form a transparent and uniform composite film. Thus, the obtained silver cluster film had an even and smooth morphology (Supplementary Fig. 35), which was conducive to further fabrication of SCLC devices. Moreover, to screen the optimal luminescent silver cluster film, a series of control experiments with different doping amounts of silver clusters were conducted (Supplementary Fig. 36), and the emission intensities increased and then decreased with increasing R-Ag6 weight, resulting in 10 mg of R-Ag6 being the optimal doping amount for subsequent SCLC device research. The temperature-dependent photoluminescence spectra of the thin film were measured. When the temperature increased from 77 K to 370 K, the emission peak of the R-Ag6/PMMA film exhibited a blue shift, and its emission intensity decreased (Supplementary Fig. 37). The PLQY and lifetime of the R-Ag6/PMMA film were subsequently measured, and they increased to 32% and 8.5 μs, respectively, due to inhibition of nonradiative transitions (Supplementary Fig. 38). In addition, transmission electron microscopy (TEM) demonstrated that the silver clusters (R-Ag6) were successfully embedded in the PMMA matrix, and their size was almost the same as that of R-Ag6 in the solution (Supplementary Figs. 39 and 40). To verify that the silver clusters maintain their integrity after copolymerisation, PXRD, PL and UV‒vis absorption were performed. The PXRD results revealed that there were no obvious characteristic diffraction peaks of the silver clusters in the polymer composite film, suggesting that the silver clusters existed in a discrete state within the polymer (PMMA) matrix (Supplementary Fig. 41). This conclusion was confirmed by the almost identical UV‒vis absorption and PL peaks of the silver clusters in the discrete state (DCM solution) and within the PMMA matrix (Supplementary Figs. 42).
Fig. 5: CPL behaviour of (S/R)-Ag6 in stage IV.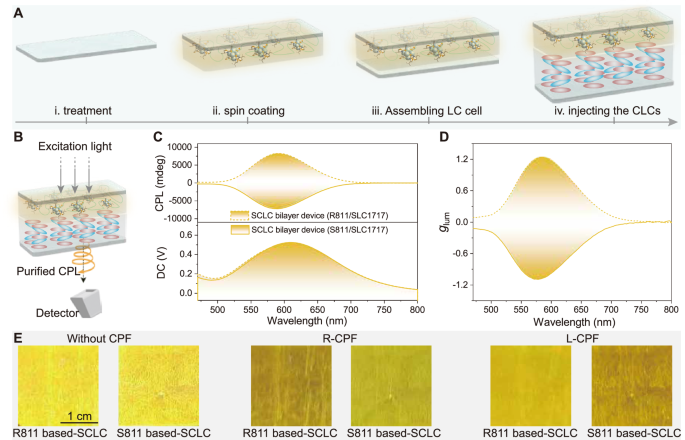
A Schematic fabrication of the SCLC devices used in this work. B Illustration of the purified CPL activity of silver clusters in the SCLC device. The excitation light first excited an SCLC device, and the CPL from silver clusters entered the detector. C CPL spectra of the SCLC devices (λex = 370 nm). D glum curves of the SCLC devices. E Luminescent images of the SCLC devices, which were captured without a circular polariser (left column) and through a right-handed circular polariser (middle column), and a left-handed circular polariser (right column).
As shown in Fig. 5A, SCLC devices were then constructed by injecting the CLC (SLC1717 and R811/S811) into an LC cell, where the PBG could be tuned by adjusting the weight ratio of R811/SLC1717 or S811/SLC1717 according to need54, namely, stage IV. Moreover, the stability of the R-Ag6/PMMA film is essential for SCLC bilayer devices. SEM images of the Ag6-embedded PMMA film and the composite film after LC injection and subsequent removal were subsequently obtained (Supplementary Figs. 43 and 44), and the results revealed that the morphology of the film did not change. Furthermore, there was no change in the elemental composition of the R-Ag6/PMMA composite film after LC injection. Together, these results indicated that the R-Ag6/PMMA films possessed high resistance to a polar LC layer. Thus, SCLC devices with a bilayer architecture could be fabricated by employing a transparent and luminescent composite film and a tunable CLC layer.
Figure 5B illustrates the purified CPL of the silver clusters in the SCLC device. Impressively, when the weight ratio of S811/SLC1717 in the CLC layer was 27 wt%, the bilayer devices exhibited intense mirror-image CPL responses near the emission peak of the silver clusters, showing a good glum factor of 1.24 (Fig. 5C, D), which is superior to all reported glum factors of coinage metal clusters to our knowledge (Fig. 1C and Supplementary Table 1). We speculated that there was better coupling between the Ag6-embedded PMMA composite film and the CLC layer in the SCLC bilayer devices because of the even and smooth composite film, whereas the silver clusters doped into the CLCs deformed the helical arrangement of the CLCs, which was why the CPL activity was poor in the ternary assemblies. In addition, the obtained glum factor was greater than those observed at the other concentrations of the chiral dopants (Supplementary Fig. 45). To corroborate the CPL effect, we then measured the PL spectra through left- and right-handed circular polarised filters, and the emission spectra exhibited significant differences because CPL with the same handedness could pass through, whereas CPL with the opposite handedness was blocked (Supplementary Fig. 46). As shown in Supplementary Figs. 47 and 48, the SCLC devices we designed not only guaranteed excellent CPL but also displayed circular polarisation characteristics visible to the naked eye (Fig. 5E), enabling the SCLC devices to achieve specific display anti-counterfeiting functions while having the potential for 3D display (Supplementary Movie 1 and Supplementary Fig. 49). These results collectively substantiated that the SCLC devices with a bilayer architecture could demonstrate high-quality CPL performance of the silver clusters.
Moreover, the CPL performance can be evaluated by the FM (FM = |glum | × PLQY)55,56. Correspondingly, we obtained the FM values for silver clusters in different stages (I, II, III, and IV), with a maximum FM value of 0.4 observed in stage IV (SCLC devices), which is also higher than those of all reported coinage metal clusters (Fig. 1C and Supplementary Table 1). As shown in Supplementary Fig. 50, the SCLC bilayer device (R-Ag6 embedded into PMMA, R811/SLC1717 CLC layer) maintained over 1.17 of the glum after 23 days, showing good long-term stability. More interestingly, in the aforementioned test method, the CLC layer was oriented towards the detector, and a high glum factor could be obtained. However, when the CLC layer faced the excitation light, inverted and weaker glum factors (+ 0.36/− 0.32) were observed without an optical aid (Supplementary Figs. 51 and 52). Considering this particular phenomenon, combined with the formula for the glum factor, in the case of the R811 based-SCLC bilayer devices, the overlap of the luminescent composite film layer and the PBG can be understood to significantly minimise the contribution of IR, thereby leading to a high glum factor, analogous to the purification of circularly polarised light (when the CLC layer faced the detector). For the S811 based-SCLC bilayer devices, the situation is reversed. When the luminescent composite film layer faced the detector, the inefficient optical design led to simultaneous contributions of IL and IR57, yielding a relatively poor glum factor.
Generality of the strategy
The universality of this strategy holds paramount importance for augmenting the chiroptical activity of metal clusters, as it would facilitate greater efficiency and diminish unnecessary duplication of efforts. To verify the universality, Au(I)-Cu(I)-doped metal clusters were subsequently investigated through the strategy mentioned above58. First, enantiomeric AuCu4 clusters were synthesised (the detailed process is described in the supporting information, Supplementary Fig. 2). R/S-AuCu4 contains four copper(I) atoms and one gold(I) atom, which are bridged by two N-heterocyclic carbene (NHC) ligands and stabilised by four I atoms (Supplementary Figs. 53 and 54). We confirmed the crystalline phase purity of the R/S-AuCu4 clusters by PXRD and examined their PL (Supplementary Figs. 55 and 56). They exhibited intense PL (PLQY, 0.43) centred at 615 nm in DCM (Supplementary Fig. 57). Moreover, according to the absorption and PL spectra, the concentration of chiral dopants in SLC1717 was adjusted so that the PBG of the CLCs covered the emission band of the enantiopure AuCu4 (Supplementary Fig. 58). As expected, compared with AuCu4 in the discrete state, AuCu4 showed enhanced CPL signals with |glum| factors of 0.57 and 1.23 in stage III and stage IV, respectively (Supplementary Figs. 59–61). From this perspective, this strategy is considered to be a versatile approach for constructing metal clusters with excellent chiroptical properties.
Enantioselective photopolymerization based on light-to-matter chirality transfer
The SCLC bilayer device (stage IV) with intense CPL signals motivated us to put it into real applications. Currently, a physical method gaining attention in asymmetric synthesis of polymers is enantioselective photopolymerization induced by CPL because of its alignment with the principles of green chemistry10,36,59,60,61,62. In addition, the introduction of CPL-active materials into CPL photocontrol of polymer entities is highly important for light-to-matter chiral transfer and chiral expression at different hierarchical levels37,38. Specifically, 10,12-pentacosadiynoic acid (DA) was dissolved in a mixture of water and ethanol (1:4) and then dropped onto a quartz plate, forming an unpolymerized DA film. An experimental setup (Supplementary Fig. 62) employing the SCLC bilayer devices to generate L-CPL or R-CPL under irradiation with a UV lamp was then used to irradiate the DA film to form chiral poly(10,12-pentacosadiynoic acid) (PDA). This process was carried out in conjunction with an unpolarised UV lamp (365 nm) and was accompanied by a colour change of the film from white to blue. Notably, the resulting PDA films exhibited an obvious absorption region and a mirror-image Cotton effect depending on the handedness of the CPL (Supplementary Fig. 63), indicating the formation of chiral PDA. However, significant CD signals were not observed for either the DA film or the PDA film polymerised with UV radiation. By examining the spectra of the PDA film sample at different rotation angles, their consistency was confirmed, thereby excluding potential interference from the artifacts on the CD signals of the chiral polymers (Supplementary Fig. 64). Furthermore, the reliability of the CD spectra was also verified by LD spectra (Supplementary Fig. 65). The enantioselectivity and the degree of polymerisation clearly depended on the handedness of the CPL and the UV light, respectively.
In addition to the above asymmetric polymerisation of achiral molecules, there is also a technique known as asymmetric resolution polymerisation of a racemic mixture25,29,30. To our knowledge, utilisation of the CPL emitted from chiral materials in this technique has not yet been realised. The chirality of polymers usually cannot be generated from racemic monomers without any chiral dopants or catalysts. Therefore, asymmetric resolution polymerisation of a racemic mixture remains a considerable challenge. Thiol-ene click chemistry63, due to its combination of absolute asymmetric synthesis and click reactions, allows enantioselective synthesis of optically active polymers from racemic monomers64. Thiol compounds are produced through the in situ ring-opening reaction of nucleophilic amine compounds since sulfhydryl groups are easily oxidised under ambient conditions65. Then, a radical thiol-ene reaction is further induced by UV light, which has been widely used in the synthesis of linear polymers. In this work, as shown in Supplementary Figs. 3 and 4, N,N-dimethylpropane-1,3-diamine was used as the ring-opening reagent for the thiolactone group of racemic N-(allyloxy)carbonyl-homocysteinethiolactone (NACHT). (i) Racemic allyl-(1-((3-(dimethylamino)-propyl)amino)-4-mercapto-1-oxobutan-2-yl)carbamate (DPAMOC) monomers containing both thiol and alkenyl groups were subsequently obtained (Supplementary Figs. 66 and 67). (ii) Thiol groups can be activated by UV light, transforming into thiyl radicals, which can then react with vinyl groups, ultimately leading to the formation of linear polymers. On the basis of process (ii), the CPL emitted from the SCLC bilayer devices was synchronously applied, with the aim of achieving symmetry breaking in the polymerisation reaction through the interactions between chiral thiyl radical intermediates and CPL, enabling enantioselective polymerisation of racemic precursors (Supplementary Fig. 68). Therefore, whether yellow CPL with a high glum factor based on silver clusters can trigger enantioselective polymerisation of thiol-ene was the focus of subsequent research.
To verify this hypothesis, we built an experimental platform, as shown in Fig. 6A. The setup involved exciting SCLC bilayer devices under irradiation with 365 nm UV light to produce yellow CPL emissions (L-CPL or R-CPL), which were used as chiral sources. The SCLC bilayer device was placed between a cuvette containing the racemic monomer DPAMOC in 1,4-dioxane and the aforementioned UV light source. After exposure to yellow CPL and additional UV irradiation (365 nm) for 100 min, the chiral linear polymers were precipitated by adding ether for further study (Fig. 6B and Supplementary Movie 2). The Fourier transform infrared (FTIR) spectra of the monomer DPAMOC and the linear polymers in Fig. 6D revealed that the stretching peak of the -SH groups at 2556 cm−1 disappeared, which strongly confirmed that thiol-ene click polymerisation between the thiol and alkenyl groups in different monomers occurred66,67. To avoid any possibility of coincidence, multiple FTIR tests were conducted on both the resulting linear polymers and the polymer monomers (Supplementary Fig. 69), and the results consistently revealed the disappearance of the -SH peak, confirming that the monomers had polymerised into linear polymers. Scanning electron microscopy (SEM) images revealed that the linear polymers had no specific morphology (Supplementary Fig. 70). More importantly, as anticipated, the resulting polymers showed a distinct mirror-image Cotton effect upon exposure to CPL with different handedness (Fig. 6C), demonstrating negative CD signals when exposed to L-CPL and normal UV light, conversely, positive CD signals. The reliability of the CD spectra was verified by excluding the possible LD effect (Supplementary Fig. 71). In addition, in the absence of CPL, the resulting linear polymers did not exhibit the Cotton effect (Supplementary Fig. 72). The CD signals originated from an excess of R-type or S-type DPAMOC monomers in the final polymer chains29, which indicated that the racemic DPAMOC monomers underwent asymmetric resolution polymerisation without any catalysts.
Fig. 6: Enantioselective photopolymerization.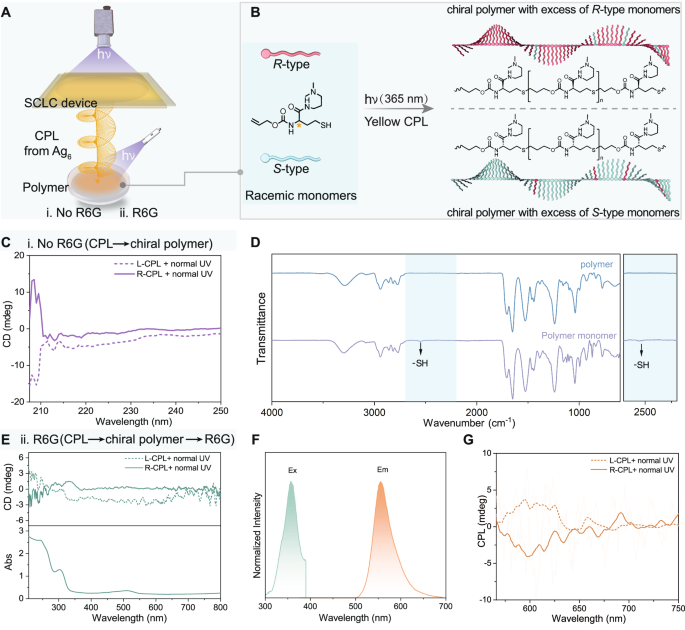
A Schematic representation of the experimental setup for the photoinduced polymerisation of DPAMOC. B Schematic illustration of the production of an optically active polymer from racemic monomers (DPAMOC) through the asymmetric thiol–ene polymerisation process triggered by the yellow CPL from silver clusters and UV irradiation at 365 nm. C C D spectra of the resultant chiral polymers prepared by photopolymerization triggered by CPL and normal UV light. Chirality transfer from CPL to the chiral polymer. D FTIR spectra of the resulting linear polymer and polymer monomer. E CD spectra of the resulting host‒guest systems prepared by photopolymerization with the addition of the dye molecule R6G. Chirality transfer from CPL to the chiral polymer to R6G. F Normalised excitation and emission spectra of the chiral polymer‒R6G host‒guest system. G CPL spectra of the resulting host‒guest systems (chiral polymer and R6G).
Chiral polymers have been widely used as hosts to induce chiroptical activity of guest materials in recent years27,68. On this basis, we introduced a CPL testing technique to further investigate the chirality of the final linear polymers. Specifically, we constructed a host‒guest system in a polymer system. Fluorescent dyes were added to the polymerisation system before the polymerisation reaction. Assuming that the resulting polymers were chiral, their chirality would be transferred to the dye molecules; therefore, the constructed host (polymer)‒guest (dye) system would generate induced chiroptical activities of the dye molecules. In the specific experiment, an ethanol solution of dye molecules (R6G, 500 μL, 10−1 M) was introduced into a polymer monomer system. After 100 min of irradiation with the CPL from the SCLC bilayer devices as well as normal UV light, the polymer–dye system was successfully constructed and impressively demonstrated significant chiroptical activities of the dye molecules (Fig. 6E–G), whether in terms of CD or CPL. These results confirmed that chirality was sequentially transferred from CPL to the polymers and then to the dye molecules, in which the polymers acted as chirality transfer hubs (Fig. 6E), indirectly confirming that chiral polymers were successfully prepared through asymmetric resolution polymerisation. Furthermore, as a contrast, the CPL from the silver clusters in stage III with a lower |glum| factor (0.4) was used as the chiral source for polymerisation instead of that of the SCLC bilayer devices, and the resulting polymer‒dye films were CPL silent (Supplementary Fig. 73). This result indicated that the glum factor of the chiral source (CPL) played a significant role in asymmetric resolution polymerisation in this work. The above results clearly revealed that enantioselective polymerisation could be realised by using CPL as the chiral source and that the induced optical activity of the resulting chiral polymer was contingent upon the handedness of the CPL.
These findings collectively and compellingly demonstrate that the chirality of polymers can be regulated by the chirality of the CPL from silver clusters, realising light-to-matter chirality transfer, which provides simplicity and versatility of chiral synthesis and deepens the understanding of the origin of homochirality in living matter.