 article
article
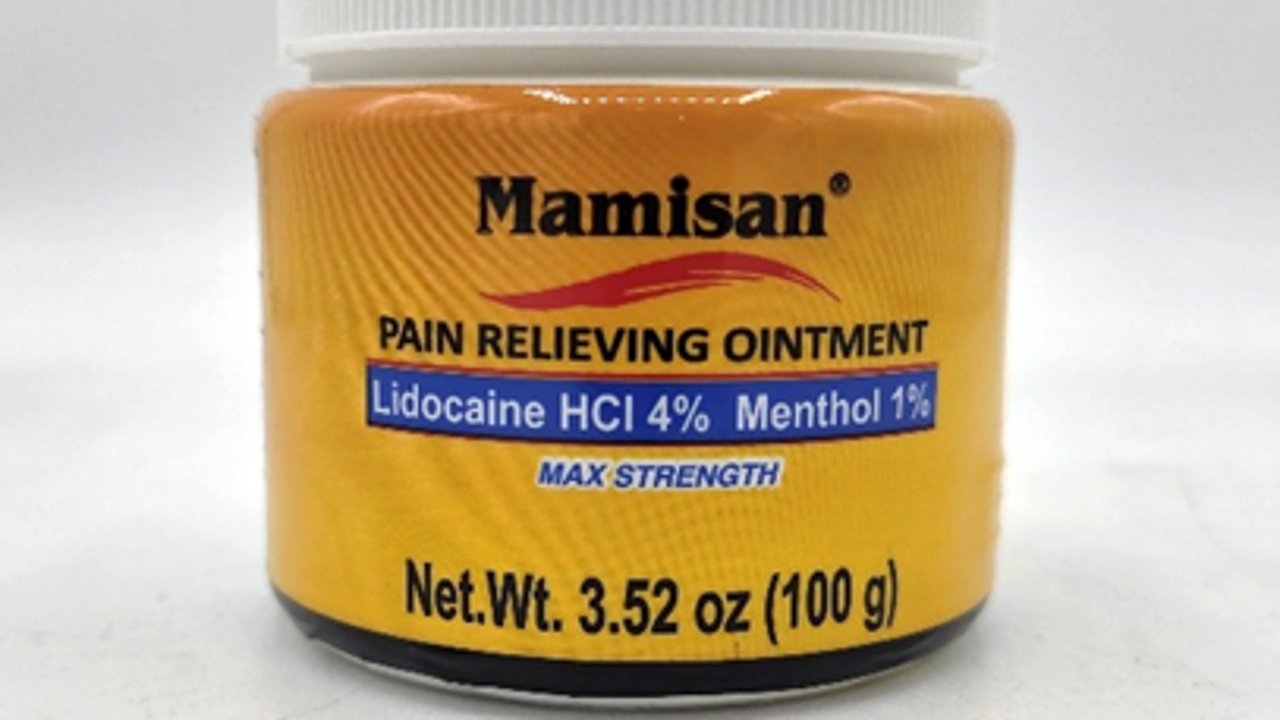
Mamisan Pain Relieving Topical Ointment. (Credit: U.S. Consumer Product Safety Commission)
Plantimex has issued a recall of its Mamisan Lidocaine Ointment Containers over concerns it can cause serious injury or death from child poisoning and violates federal safety standards.
Why you should care:
According to the U.S. Consumer Product Safety Commission, the Mamisan Pain Relieving Topical Ointment contains lidocaine and is required under the Poison Prevention Packaging Act to be sold in child-resistant packaging, but the product’s noncompliant container poses a risk of serious injury or death if ingested by young children.
About 50,330 units are affected by the recall.
RELATED: Ford recalls more than 270,000 electric, hybrid vehicles due to roll-away risk
So far, no injuries have been reported.
Dig deeper:
The recall covers Mamisan Pain Relieving Topical Ointment sold in 3.52-ounce orange plastic jars with a white screw-top lid and Mamisan branding, specifically those bearing UPC code 860006498115.
RELATED: Blue Wave recalls over 13,000 above-ground pools due to drowning risk
It was sold at Walmart and Target stores nationwide and online at Target.com from April 2024 through October 2025 for about $10.
What you can do:
Consumers should immediately keep the recalled ointment out of children’s sight and reach and contact Plantimex for a free replacement lid, after which the product may be used as directed. Plantimex toll-free at 855-752-6869 from 9 a.m. to 4 p.m. PT Monday through Friday, email at customercare@plantimexusa.com with the subject “RECALL” or online at http://plantimexusa.com/contact.php or www.plantimexusa.com.
The Source: Information in this story is based on a recall notice issued by the U.S. Consumer Product Safety Commission. This story was reported from Los Angeles.