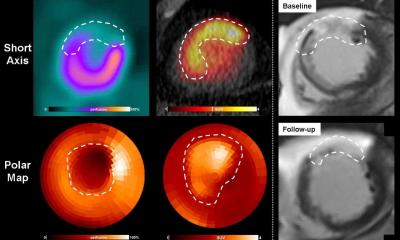
Conventional imaging modalities, including MPI and cardiac MR, predominantly quantify the extent of irreversible tissue damage but do not capture the dynamic inflammatory response that governs healing
Johanna Diekmann
Researchers speculated that CXCR4 upregulation early after AMI would predict left ventricular remodeling in the heart as well as cardiac structural functional outcome. To test this hypothesis, they performed comprehensive multimodal evaluations with CXCR4-targeted PET/CT, myocardial perfusion imaging (MPI), and cardiac MRI on 49 patients within the first week after AMI. Follow-up cardiac MRI was also acquired approximately eight months after AMI in 40 of the patients.
The integrative approach demonstrated that CXCR4 upregulation extends beyond the infarct core, involves the border zone, and correlates with subsequent left ventricular dysfunction. These findings offer mechanistic insight into the link between post-ischemic inflammation and remodeling and underscores the clinical relevance of inflammatory activity after AMI.
“Conventional imaging modalities, including MPI and cardiac MR, predominantly quantify the extent of irreversible tissue damage but do not capture the dynamic inflammatory response that governs healing,” noted Diekmann. “By including CXCR4-targeted PET, we can identify patients who exhibit excessive or prolonged inflammation which may predispose them to adverse remodeling and heart failure. Such information could, in the future, support risk stratification and guide emerging anti-inflammatory or reparative therapies in a precision medicine framework.”
“What’s more,” she continued, “this approach may ultimately facilitate image-guided therapeutic strategies, allowing nuclear medicine to play an active role in monitoring and optimizing interventions that modulate inflammation, repair, and regeneration after cardiac injury.”
Source: Society of Nuclear Medicine and Molecular Imaging