Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY). 2016;8:3–11.
d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–22.
Erusalimsky JD. Oxidative stress, telomeres and cellular senescence: what non-drug interventions might break the link? Free Radic Biol Med. 2020;150:87–95.
Liu XL, Ding J, Meng LH. Oncogene-induced senescence: a double edged sword in cancer. Acta Pharmacol Sin. 2018;39:1553–8.
Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9:645593.
Liu B, Peng Z, Zhang H, Zhang N, Liu Z, Xia Z, et al. Regulation of cellular senescence in tumor progression and therapeutic targeting: mechanisms and pathways. Mol Cancer. 2025;24:106.
Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149(3):1269–83.
Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–30.
Galanos P, Vougas K, Walter D, Polyzos A, Maya-Mendoza A, Haagensen EJ, et al. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat Cell Biol. 2016;18:777–89.
Milanovic M, Fan DNY, Belenki D, Dabritz JHM, Zhao Z, Yu Y, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553(2018):96–100.
Zampetidis CP, Galanos P, Angelopoulou A, Zhu Y, Polyzou A, Karamitros T, et al. A recurrent chromosomal inversion suffices for driving escape from oncogene-induced senescence via subTAD reorganization. Mol Cell. 2021;81:4907-4923 e4908.
Herbstein F, Sapochnik M, Attorresi A, Pollak C, Senin S, Gonilski-Pacin D, et al. The SASP factor IL-6 sustains cell-autonomous senescent cells via a cGAS-STING-NFkappaB intracrine senescent noncanonical pathway. Aging Cell. 2024;23:e14258.
Ortiz-Montero P, Londono-Vallejo A, Vernot JP. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal. 2017;15:17.
Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34:1565–76.
Young AR, Narita M. SASP reflects senescence. EMBO Rep. 2009;10(2009):228–30.
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. Cellular senescence: defining a path forward. Cell. 2019;179(2019):813–27.
Kohli J, Wang B, Brandenburg SM, Basisty N, Evangelou K, Varela-Eirin M, et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat Protoc. 2021;16(2021):2471–98.
Ogrodnik M, Carlos Acosta J, Adams PD, d’Adda di Fagagna F, Baker DJ, Bishop CL, Chandra T, Collado M, Gil J, Gorgoulis V, Gruber F, Hara E, Jansen-Dürr P, Jurk D, Khosla S, Kirkland JL, Krizhanovsky V, Minamino T, Niedernhofer LJ, Passos JF, Ring NAR, Redl H, Robbins PD, Rodier F, Scharffetter-Kochanek K, Sedivy JM, Sikora E, Witwer K, von Zglinicki T, Yun MH, Grillari J, Demaria M. Guidelines for minimal information on cellular senescence experimentation in vivo. Cell. 2024;187:4150-4175.
Magkouta S, Veroutis D, Pousias A, Papaspyropoulos A, Pippa N, Lougiakis N, et al. A fluorophore-conjugated reagent enabling rapid detection, isolation and live tracking of senescent cells. Mol Cell. 2023;83:3558-3573 e3557.
Magkouta S, Veroutis D, Pousias A, Papaspyropoulos A, Giannetti K, Pippa N, et al. One-step rapid tracking and isolation of senescent cells in cellular systems, tissues, or animal models via GLF16. STAR Protoc. 2024;5:102929.
Fitsiou E, Soto-Gamez A, Demaria M. Biological functions of therapy-induced senescence in cancer. Semin Cancer Biol. 2022;81:5–13.
Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288:518–36.
Carpenter VJ, Saleh T, Gewirtz DA. Senolytics for cancer therapy: is all that glitters really gold? Cancers (Basel). 2021;13(4):723. https://doi.org/10.3390/cancers13040723.
Vernot JP. Senescence-associated pro-inflammatory cytokines and tumor cell plasticity. Front Mol Biosci. 2020;7:63.
Faget DV, Ren Q, Stewart SA. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer. 2019;19:439–53.
Pukhalskaia TV, Yurakova TR, Bogdanova DA, Demidov ON. Tumor-associated senescent macrophages, their markers, and their role in tumor microenvironment. Biochemistry (Mosc). 2024;89:839–52.
Takasugi M, Yoshida Y, Hara E, Ohtani N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 2023;290(1348):1348–61.
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68.
Silva EM, Mariano VS, Pastrez PRA, Pinto MC, Castro AG, Syrjanen KJ, et al. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS One. 2017;12:e0181125.
Felcher CM, Bogni ES, Kordon EC. Il-6 cytokine family: a putative target for breast cancer prevention and treatment. Int J Mol Sci. 2022;23(3):1809. https://doi.org/10.3390/ijms23031809.
Bent EH, Gilbert LA, Hemann MT. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev. 2016;30:1811–21.
Drost EM, MacNee W. Potential role of IL-8, platelet-activating factor and TNF-alpha in the sequestration of neutrophils in the lung: effects on neutrophil deformability, adhesion receptor expression, and chemotaxis. Eur J Immunol. 2002;32:393–403.
Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833–41.
Chambers CR, Ritchie S, Pereira BA, Timpson P. Overcoming the senescence-associated secretory phenotype (SASP): a complex mechanism of resistance in the treatment of cancer. Mol Oncol. 2021;15(2):3242–55.
Jeong Y, Yoon SY, Jung SP, Nam SJ, Lee JE, Kim S. Inhibition of interleukin-8/C-X-C chemokine receptor 2 signaling axis prevents tumor growth and metastasis in triple-negative breast cancer cells. Pharmacology. 2025;110:178–90.
Prattichizzo F, Giuliani A, Recchioni R, Bonafe M, Marcheselli F, De Carolis S, et al. Anti-TNF-alpha treatment modulates SASP and SASP-related microRNAs in endothelial cells and in circulating angiogenic cells. Oncotarget. 2016;7:11945–58.
Dong Z, Luo Y, Yuan Z, Tian Y, Jin T, Xu F. Cellular senescence and SASP in tumor progression and therapeutic opportunities. Mol Cancer. 2024;23:181.
Parajuli P, Rosati R, Mamdani H, Wright RE 3rd, Hussain Z, Naeem A, et al. Senescence-associated secretory proteins induced in lung adenocarcinoma by extended treatment with dexamethasone enhance migration and activation of lymphocytes. Cancer Immunol Immunother. 2023;72:1273–84.
Kawagoe Y, Kawashima I, Sato Y, Okamoto N, Matsubara K, Kawamura K. Cxcl5-Cxcr2 signaling is a senescence-associated secretory phenotype in preimplantation embryos. Aging Cell. 2020;19:e13240.
Huda N, Khambu B, Liu G, Nakatsumi H, Yan S, Chen X, et al. Senescence connects autophagy deficiency to inflammation and tumor progression in the liver. Cell Mol Gastroenterol Hepatol. 2022;14:333–55.
Soto-Gamez A, van Es M, Hageman E, Serna-Salas SA, Moshage H, Demaria M, et al. Mesenchymal stem cell-derived HGF attenuates radiation-induced senescence in salivary glands via compensatory proliferation. Radiother Oncol. 2024;190:109984.
Siraj Y, Aprile D, Alessio N, Peluso G, Di Bernardo G, Galderisi U. IGFBP7 is a key component of the senescence-associated secretory phenotype (SASP) that induces senescence in healthy cells by modulating the insulin IGF, and activin A pathways. Cell Commun Signal. 2024;22:540.
von Joest M, Chen C, Douche T, Chantrel J, Chiche A, Gianetto QG, et al. Amphiregulin mediates non-cell-autonomous effect of senescence on reprogramming. Cell Rep. 2022;40:111074.
Wang C, Long Q, Fu Q, Xu Q, Fu D, Li Y, et al. Targeting epiregulin in the treatment-damaged tumor microenvironment restrains therapeutic resistance. Oncogene. 2022;41:4941–59.
Guo Y, Ayers JL, Carter KT, Wang T, Maden SK, Edmond D, et al. Senescence-associated tissue microenvironment promotes colon cancer formation through the secretory factor GDF15. Aging Cell. 2019;18:e13013.
Tominaga K, Suzuki HI. TGF-beta signaling in cellular senescence and aging-related pathology. Int J Mol Sci. 2019;20(20):5002. https://doi.org/10.3390/ijms20205002 .
Wang N, Fang Y, Hou Y, Cheng D, Dressler EV, Wang H, et al. Senescent cells promote breast cancer cells motility by secreting GM-CSF and bFGF that activate the JNK signaling pathway. Cell Commun Signal. 2024;22:478.
Alessio N, Squillaro T, Di Bernardo G, Galano G, De Rosa R, Melone MAB, et al. Increase of circulating IGFBP-4 following genotoxic stress and its implication for senescence. Elife. 2020;9:e54523. https://doi.org/10.7554/eLife.54523.
Evans DS, Young D, Tanaka T, Basisty N, Bandinelli S, Ferrucci L, et al. Proteomic analysis of the senescence-associated secretory phenotype: GDF-15, IGFBP-2, and cystatin-C are associated with multiple aging traits. J Gerontol A Biol Sci Med Sci. 2024;79(3):glad265 https://doi.org/10.1093/gerona/glad265.
Xu Q, Long Q, Zhu D, Fu D, Zhang B, Han L, et al. Targeting amphiregulin (AREG) derived from senescent stromal cells diminishes cancer resistance and averts programmed cell death 1 ligand (PD-L1)-mediated immunosuppression. Aging Cell. 2019;18:e13027.
Strzyz P. Senescent fibroblasts support lung repair. Nat Rev Mol Cell Biol. 2022;23:775.
Alexander PB, Yuan L, Yang P, Sun T, Chen R, Xiang H, et al. EGF promotes mammalian cell growth by suppressing cellular senescence. Cell Res. 2015;25:135–8.
Shang D, Sun D, Shi C, Xu J, Shen M, Hu X, et al. Activation of epidermal growth factor receptor signaling mediates cellular senescence induced by certain pro-inflammatory cytokines. Aging Cell. 2020;19:e13145.
Vassilieva I, Kosheverova V, Vitte M, Kamentseva R, Shatrova A, Tsupkina N, et al. Paracrine senescence of human endometrial mesenchymal stem cells: a role for the insulin-like growth factor binding protein 3. Aging (Albany NY). 2020;12(2):1987–2004.
Hu D, Ge Y, Cui Y, Li K, Chen J, Zhang C, et al. Upregulated IGFBP3 with aging is involved in modulating apoptosis, oxidative stress, and fibrosis: a target of age-related erectile dysfunction. Oxid Med Cell Longev. 2022;2022:6831779.
Chen Q, Tang L, Zhang Y, Wan C, Yu X, Dong Y, et al. STING up-regulates VEGF expression in oxidative stress-induced senescence of retinal pigment epithelium via NF-kappaB/HIF-1alpha pathway. Life Sci. 2022;293:120089.
Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97(2):978–85.
Menicacci B, Laurenzana A, Chilla A, Margheri F, Peppicelli S, Tanganelli E, et al. Chronic resveratrol treatment inhibits MRC5 fibroblast SASP-related protumoral effects on melanoma cells. J Gerontol A Biol Sci Med Sci. 2017;72:1187–95.
Hasan MR, Ho SH, Owen DA, Tai IT. Inhibition of VEGF induces cellular senescence in colorectal cancer cells. Int J Cancer. 2011;129(2011):2115–23.
Alqahtani S, Alqahtani T, Venkatesan K, Sivadasan D, Ahmed R, Sirag N, Elfadil H, Abdullah Mohamed H, T A H, Elsayed Ahmed R, Muralidharan P, Paulsamy P. SASP modulation for cellular rejuvenation and tissue homeostasis: therapeutic strategies and molecular insights. Cells. 2025;14.(8):608. https://doi.org/10.3390/cells14080608.
Jiang B, Zhang W, Zhang X, Sun Y. Targeting senescent cells to reshape the tumor microenvironment and improve anticancer efficacy. Semin Cancer Biol. 2024;101:58–73.
Zhao S, Qiao Z, Pfeifer R, Pape HC, Mao K, Tang H, et al. Modulation of fracture healing by senescence-associated secretory phenotype (SASP): a narrative review of the current literature. Eur J Med Res. 2024;29:38.
Jakhar R, Crasta K. Exosomes as emerging pro-tumorigenic mediators of the senescence-associated secretory phenotype. Int J Mol Sci. 2019;20(10):2547. https://doi.org/10.3390/ijms20102547.
DeClerck YA, Mercurio AM, Stack MS, Chapman HA, Zutter MM, Muschel RJ, et al. Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol. 2004;164:1131–9.
Mebratu YA, Soni S, Rosas L, Rojas M, Horowitz JC, Nho R. The aged extracellular matrix and the profibrotic role of senescence-associated secretory phenotype. Am J Physiol Cell Physiol. 2023;325:C565-79.
Hassona Y, Cirillo N, Heesom K, Parkinson EK, Prime SS. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br J Cancer. 2014;111:1230–7.
Lan YY, Chang FH, Tsai JH, Chang Y. Epstein-barr virus Rta promotes invasion of bystander tumor cells through paracrine of matrix metalloproteinase 9. Biochem Biophys Res Commun. 2018;503:2160–6.
Ozcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY). 2016;8:1316–29.
Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–63.
Rana T, Jiang C, Liu G, Miyata T, Antony V, Thannickal VJ, et al. PAI-1 regulation of TGF-beta1-induced alveolar type II cell senescence, SASP secretion, and SASP-mediated activation of alveolar macrophages. Am J Respir Cell Mol Biol. 2020;62(319):319–30.
Wu J, Wang J, Pei Z, Zhu Y, Zhang X, Zhou Z, et al. Endothelial senescence induced by PAI-1 promotes endometrial fibrosis. Cell Death Discov. 2025;11:89.
Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002;294:1155–60.
Giroud J, Bouriez I, Paulus H, Pourtier A, Debacq-Chainiaux F, Pluquet O. Exploring the communication of the SASP: dynamic, interactive, and adaptive effects on the microenvironment. Int J Mol Sci. 2023;24(13):10788. https://doi.org/10.3390/ijms241310788.
Dasgupta N, Arnold R, Equey A, Gandhi A, Adams PD. The role of the dynamic epigenetic landscape in senescence: orchestrating SASP expression. NPJ Aging. 2024;10:48.
Ou HL, Schumacher B. DNA damage responses and p53 in the aging process. Blood. 2018;131:488–95.
Lopez AR, Jorgensen MH, Havelund JF, Arendrup FS, Kolapalli SP, Nielsen TM, et al. Autophagy-mediated control of ribosome homeostasis in oncogene-induced senescence. Cell Rep. 2023;42:113381.
Faheem MM, Seligson ND, Ahmad SM, Rasool RU, Gandhi SG, Bhagat M, et al. Convergence of therapy-induced senescence (TIS) and EMT in multistep carcinogenesis: current opinions and emerging perspectives. Cell Death Discov. 2020;6:51.
Ye T, Gao H, Zhang ZR, Ge Y, Liu YK, Yan JY, et al. Epigenetic regulation of cellular senescence in gastrointestinal cancer. Mol Cancer Ther. 2025. https://doi.org/10.1158/1535-7163.MCT-24-0949.
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–7.
Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–42.
Sheekey E, Narita M. P53 in senescence – it’s a marathon, not a sprint. FEBS J. 2023;290(3):1212–20.
Zhao J, Zhang L, Lu A, Han Y, Colangelo D, Bukata C, et al. ATM is a key driver of NF-kappaB-dependent DNA-damage-induced senescence, stem cell dysfunction and aging. Aging (Albany NY). 2020;12:4688–710.
Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–43.
Cheng Q, Chen J. Mechanism of p53 stabilization by ATM after DNA damage. Cell Cycle. 2010;9:472–8.
Manic G, Obrist F, Sistigu A, Vitale I. Trial watch: targeting ATM-CHK2 and ATR-CHK1 pathways for anticancer therapy. Mol Cell Oncol. 2015;2:e1012976.
Engeland K. Cell cycle regulation: p53–p21-RB signaling. Cell Death Differ. 2022;29:946–60.
Kim YY, Jee HJ, Um JH, Kim YM, Bae SS, Yun J. Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell. 2017;16(2017):1094–103.
Yan J, Chen S, Yi Z, Zhao R, Zhu J, Ding S, et al. The role of p21 in cellular senescence and aging-related diseases. Mol Cells. 2024;47:100113.
Gire V, Roux P, Wynford-Thomas D, Brondello JM, Dulic V. DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J. 2004;23:2554–63.
Aasland D, Gotzinger L, Hauck L, Berte N, Meyer J, Effenberger M, et al. Temozolomide induces senescence and repression of DNA repair pathways in glioblastoma cells via activation of ATR-CHK1, p21, and NF-kappaB. Cancer Res. 2019;79:99–113.
Safwan-Zaiter H, Wagner KD, Wagner N. P16INK4A-more than a senescence marker. Life. 2022;12(9):1332. https://doi.org/10.3390/life12091332.
Ohtani N, Yamakoshi K, Takahashi A, Hara E. The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J Med Invest. 2004;51:146–53.
Gimenez-Bastida JA, Avila-Galvez MA, Espin JC, Gonzalez-Sarrias A. Conjugated physiological resveratrol metabolites induce senescence in breast cancer cells: role of p53/p21 and p16/Rb pathways, and ABC transporters. Mol Nutr Food Res. 2019;63:e1900629.
Chen J, Huang X, Halicka D, Brodsky S, Avram A, Eskander J, et al. Contribution of p16INK4a and p21CIP1 pathways to induction of premature senescence of human endothelial cells: permissive role of p53. Am J Physiol Heart Circ Physiol. 2006;290:H1575-1586.
Rezatabar S, Karimian A, Rameshknia V, Parsian H, Majidinia M, Kopi TA, et al. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J Cell Physiol. 2019;234:14951–65.
Roy S, Roy S, Rana A, Akhter Y, Hande MP, Banerjee B. The role of p38 MAPK pathway in p53 compromised state and telomere mediated DNA damage response. Mutat Res-Genet Toxicol Environ Mutagen. 2018;836:89–97.
Xu L, Ma Y, Ji Y, Ma Y, Wang Y, Zhao X, et al. Obesity exacerbates postoperative cognitive dysfunction by activating the PARP1/NAD(+)/SIRT1 axis through oxidative stress. Exp Gerontol. 2023:112320. https://doi.org/10.1016/j.exger.2023.112320.
Murata MM, Kong X, Moncada E, Chen Y, Imamura H, Wang P, et al. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol Biol Cell. 2019;30:2584–97.
Mendelsohn AR, Larrick JW. The NAD+/PARP1/SIRT1 axis in aging. Rejuvenation Res. 2017;20:244–7.
Sfikas A, Batsi C, Tselikou E, Vartholomatos G, Monokrousos N, Pappas P, et al. The canonical NF-kappaB pathway differentially protects normal and human tumor cells from ROS-induced DNA damage. Cell Signal. 2012;24:2007–23.
Jing H, Lee S. NF-kappaB in cellular senescence and cancer treatment. Mol Cells. 2014;37:189–95.
Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 2012;24:835–45.
Sebastian T, Malik R, Thomas S, Sage J, Johnson PF. C/EBPbeta cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J. 2005;24:3301–12.
Liu X, Huang Z, Zhang Y, Shui X, Liu F, Wu Z, et al. Lacidipine ameliorates the endothelial senescence and inflammatory injury through CXCR7/P38/C/EBP-beta signaling pathway. Front Cardiovasc Med. 2021;8:692540.
Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R, et al. Targeted regulation of PI3K/Akt/mTOR/NF-kappaB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anticancer Agents Med Chem. 2013;13:1002–13.
Shi T, Dansen TB. Reactive oxygen species induced p53 activation: DNA damage, redox signaling, or both? Antioxid Redox Signal. 2020;33(2020):839–59.
Guterres FA, Martinez GR, Rocha ME, Winnischofer SM. Simvastatin rises reactive oxygen species levels and induces senescence in human melanoma cells by activation of p53/p21 pathway. Exp Cell Res. 2013;319(2013):2977–88.
Zhou Z, Yin Y, Chang Q, Sun G, Lin J, Dai Y. Downregulation of B-myb promotes senescence via the ROS-mediated p53/p21 pathway, in vascular endothelial cells. Cell Prolif. 2017;50(2):e12319. https://doi.org/10.1111/cpr.12319.
Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K, et al. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol. 2006;8:1291–7.
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–22.
Zhang L, Fang Y, Xu XF, Jin DY. Moscatilin induces apoptosis of pancreatic cancer cells via reactive oxygen species and the JNK/SAPK pathway. Mol Med Rep. 2017;15:1195–203.
Wagner KD, Wagner N. The senescence markers p16INK4A, p14ARF/p19ARF, and p21 in organ development and homeostasis. Cells. 2022;11(12):1966. https://doi.org/10.3390/cells11121966.
Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey R, Papaconstantinou J. The ASK1-signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging (Albany NY). 2010;2:597–611.
Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–5.
Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642.
Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4.
Mallette FA, Ferbeyre G. The DNA damage signaling pathway connects oncogenic stress to cellular senescence. Cell Cycle. 2007;6:1831–6.
Maya-Mendoza A, Ostrakova J, Kosar M, Hall A, Duskova P, Mistrik M, et al. Myc and Ras oncogenes engage different energy metabolism programs and evoke distinct patterns of oxidative and DNA replication stress. Mol Oncol. 2015;9:601–16.
Shtutman M, Chang BD, Schools GP, Broude EV. Cellular model of p21-induced senescence. Methods Mol Biol. 2017;1534:31–9.
Xu Y, Li N, Xiang R, Sun P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci. 2014;39:268–76.
Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–48.
Ruiz L, Traskine M, Ferrer I, Castro E, Leal JF, Kaufman M, et al. Characterization of the p53 response to oncogene-induced senescence. PLoS One. 2008;3:e3230.
Patel PL, Suram A, Mirani N, Bischof O, Herbig U. Derepression of hTERT gene expression promotes escape from oncogene-induced cellular senescence. Proc Natl Acad Sci U S A. 2016;113:E5024-5033.
Yu Y, Schleich K, Yue B, Ji S, Lohneis P, Kemper K, et al. Targeting the senescence-overriding cooperative activity of structurally unrelated H3K9 demethylases in melanoma. Cancer Cell. 2018;33:322–36 e328.
Laberge RM, Awad P, Campisi J, Desprez PY. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 2012;5:39–44.
Burns TF, Dobromilskaya I, Murphy SC, Gajula RP, Thiyagarajan S, Chatley SN, et al. Inhibition of TWIST1 leads to activation of oncogene-induced senescence in oncogene-driven non-small cell lung cancer. Mol Cancer Res. 2013;11:329–38.
Cuollo L, Antonangeli F, Santoni A, Soriani A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology. 2020;9(12):485. https://doi.org/10.3390/biology9120485.
Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76:947–57.
Saleh T, Bloukh S, Carpenter VJ, Alwohoush E, Bakeer J, Darwish S, et al. Therapy-induced senescence: an “Old” friend becomes the enemy. Cancers (Basel). 2020;12(4):822. https://doi.org/10.3390/cancers12040822.
Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17:1205–17.
Loo TM, Miyata K, Tanaka Y, Takahashi A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020;111:304–11.
Yu L, Liu P. cGAS/STING signalling pathway in senescence and oncogenesis. Semin Cancer Biol. 2024;106–107:87–102.
Zeng PH, Yin WJ. The cGAS/STING signaling pathway: a cross-talk of infection, senescence and tumors. Cell Cycle. 2023;22:38–56.
Muela-Zarzuela I, Suarez-Rivero JM, Gallardo-Orihuela A, Wang C, Izawa K, de Gregorio-Procopio M, et al. Nlrp1 inflammasome promotes senescence and senescence-associated secretory phenotype. Inflamm Res. 2024;73:1253–66.
Vaissiere T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659:40–8.
Rodríguez S, Bermúdez LG, González D, Bernal C, Cañas A, Morales-Ruíz T, et al. Transcriptional regulation of CDKN2A/p16 by sirtuin 7 in senescence. Mol Med Rep. 2022;26(5):345. https://doi.org/10.3892/mmr.2022.12861.
Ito T, Teo YV, Evans SA, Neretti N, Sedivy JM. Regulation of cellular senescence by polycomb chromatin modifiers through distinct DNA damage- and histone methylation-dependent pathways. Cell Rep. 2018;22:3480–92.
Zhang N, Shang M, Li H, Wu L, Dong M, Huang B, et al. Dual inhibition of H3K9me2 and H3K27me3 promotes tumor cell senescence without triggering the secretion of SASP. Int J Mol Sci. 2022;23(7):3911. https://doi.org/10.3390/ijms23073911.
Shvedunova M, Akhtar A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. 2022;23:329–49.
Gadecka A, Koblowska M, Kossowska H, Iwanicka-Nowicka R, Janiszewska D, Mosieniak G, et al. Senescence-associated alterations in histone H3 modifications, HP1 alpha levels and distribution, and in the transcriptome of vascular smooth muscle cells in different types of senescence. Cell Commun Signal. 2025;23(1):321.
Kaneda A, Fujita T, Anai M, Yamamoto S, Nagae G, Morikawa M, et al. Activation of Bmp2-Smad1 signal and its regulation by coordinated alteration of H3K27 trimethylation in Ras-induced senescence. PLoS Genet. 2011;7:e1002359.
Warnon C, Bouhjar K, Ninane N, Verhoyen M, Fattaccioli A, Fransolet M, et al. Hdac2 and 7 down-regulation induces senescence in dermal fibroblasts. Aging (Albany NY). 2021;13:17978–8005.
Di Giorgio E, Paluvai H, Dalla E, Ranzino L, Renzini A, Moresi V, et al. Hdac4 degradation during senescence unleashes an epigenetic program driven by AP-1/p300 at selected enhancers and super-enhancers. Genome Biol. 2021;22:129.
Willis-Martinez D, Richards HW, Timchenko NA, Medrano EE. Role of HDAC1 in senescence, aging, and cancer. Exp Gerontol. 2010;45(2010):279–85.
Kupis W, Palyga J, Tomal E, Niewiadomska E. The role of sirtuins in cellular homeostasis. J Physiol Biochem. 2016;72:371–80.
Schiedel M, Robaa D, Rumpf T, Sippl W, Jung M. The current state of NAD(+) -dependent histone deacetylases (Sirtuins) as novel therapeutic targets. Med Res Rev. 2018;38:147–200.
Tasselli L, Zheng W, Chua KF. SIRT6: novel mechanisms and links to aging and disease. Trends Endocrinol Metab. 2017;28:168–85.
Tang L, Nogales E, Ciferri C. Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription. Prog Biophys Mol Biol. 2010;102(2010):122–8.
Hao F, Zhang Y, Hou J, Zhao B. Chromatin remodeling and cancer: the critical influence of the SWI/SNF complex. Epigenetics Chromatin. 2025;18:22.
Tu Z, Zhuang X, Yao YG, Zhang R. BRG1 is required for formation of senescence-associated heterochromatin foci induced by oncogenic RAS or BRCA1 loss. Mol Cell Biol. 2013;33:1819–29.
Corpet A, Stucki M. Chromatin maintenance and dynamics in senescence: a spotlight on SAHF formation and the epigenome of senescent cells. Chromosoma. 2014;123:423–36.
Olan I, Handa T, Narita M. Beyond SAHF: an integrative view of chromatin compartmentalization during senescence. Curr Opin Cell Biol. 2023;83:102206.
Yamakuchi M, Lowenstein CJ. Mir-34, SIRT1 and p53: the feedback loop. Cell Cycle. 2009;8:712–5.
Disayabutr S, Kim EK, Cha SI, Green G, Naikawadi RP, Jones KD, et al. MiR-34 mirnas regulate cellular senescence in type II alveolar epithelial cells of patients with idiopathic pulmonary fibrosis. PLoS One. 2016;11:e0158367.
Wang Y, Chen J, Chen X, Jiang F, Sun Y, Pan Y, et al. Mir-34a suppresses HNSCC growth through modulating cell cycle arrest and senescence. Neoplasma. 2017;64:543–53.
Song Y, Wang R, Li LW, Liu X, Wang YF, Wang QX, et al. Long non-coding RNA HOTAIR mediates the switching of histone H3 lysine 27 acetylation to methylation to promote epithelial-to-mesenchymal transition in gastric cancer. Int J Oncol. 2019;54:77–86.
LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res. 2014;12:167–83.
Wang K, Liu H, Hu Q, Wang L, Liu J, Zheng Z, et al. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct Target Ther. 2022;7:374.
Salminen A, Kaarniranta K, Kauppinen A. Insulin/IGF-1 signaling promotes immunosuppression via the STAT3 pathway: impact on the aging process and age-related diseases. Inflamm Res. 2021;70:1043–61.
Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell Signal. 2010;22(2):573–7.
Yan H, Lu W, Wang F. The cGAS-STING pathway: a therapeutic target in chromosomally unstable cancers. Signal Transduct Target Ther. 2023;8:45.
Samson N, Ablasser A. The cGAS-STING pathway and cancer. Nat Cancer. 2022;3:1452–63.
Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548–69.
Coombes MM, Briggs KL, Bone JR, Clayman GL, El-Naggar AK, Dent SY. Resetting the histone code at CDKN2A in HNSCC by inhibition of DNA methylation. Oncogene. 2003;22:8902–11.
Juengel E, Dauselt A, Makarevic J, Wiesner C, Tsaur I, Bartsch G, et al. Acetylation of histone H3 prevents resistance development caused by chronic mTOR inhibition in renal cell carcinoma cells. Cancer Lett. 2012;324:83–90.
Chen M, Fang Y, Liang M, Zhang N, Zhang X, Xu L, et al. The activation of mTOR signalling modulates DNA methylation by enhancing DNMT1 translation in hepatocellular carcinoma. J Transl Med. 2023;21:276.
Sergi C, Shen F, Liu SM. Insulin/IGF-1R, SIRT1, and FOXOs pathways-an intriguing interaction platform for bone and osteosarcoma. Front Endocrinol (Lausanne). 2019;10:93.
Zhang X, Zhuang M, Zhang H, Zhu Y, Yang J, Wu X, et al. Melatonin-mediated cGAS-STING signal in senescent macrophages promote TNBC chemotherapy resistance and drive the SASP. J Biol Chem. 2025;301:108438.
Ji Y, Li B, Lin R, Yuan J, Han Y, Du Y, et al. Super-enhancers in tumors: unraveling recent advances in their role in oncogenesis and the emergence of targeted therapies. J Transl Med. 2025;23:98.
Tasdemir N, Banito A, Roe JS, Alonso-Curbelo D, Camiolo M, Tschaharganeh DF, et al. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 2016;6(6):612–29.
Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599.
Valerio HP, Ravagnani FG, Ronsein GE, Di Mascio P. A single dose of Ultraviolet-A induces proteome remodeling and senescence in primary human keratinocytes. Sci Rep. 2021;11:23355.
WalderaLupa DM, Kalfalah F, Safferling K, Boukamp P, Poschmann G, Volpi E, et al. Characterization of skin aging-associated secreted proteins (SAASP) produced by dermal fibroblasts isolated from intrinsically aged human skin. J Invest Dermatol. 2015;135:1954–68.
Okamura K, Sato M, Suzuki T, Nohara K. Inorganic arsenic exposure-induced premature senescence and senescence-associated secretory phenotype (SASP) in human hepatic stellate cells. Toxicol Appl Pharmacol. 2022;454:116231.
Cohn RL, Gasek NS, Kuchel GA, Xu M. The heterogeneity of cellular senescence: insights at the single-cell level. Trends Cell Biol. 2023;33:9–17.
Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol. 2015;194:438–45.
Xiao L, Li X, Cao P, Fei W, Zhou H, Tang N, et al. Interleukin-6 mediated inflammasome activation promotes oral squamous cell carcinoma progression via JAK2/STAT3/Sox4/NLRP3 signaling pathway. J Exp Clin Cancer Res. 2022;41(1):166.
Weng YS, Tseng HY, Chen YA, Shen PC, Al Haq AT, Chen LM, et al. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Cancer. 2019;18:42.
Fu XL, Duan W, Su CY, Mao FY, Lv YP, Teng YS, et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother. 2017;66:1597–608.
Du H, Rose JP, Bons J, Guo L, Valentino TR, Wu F, et al. Substrate stiffness dictates unique doxorubicin-induced senescence-associated secretory phenotypes and transcriptomic signatures in human pulmonary fibroblasts. Geroscience. 2025;47:3941–63.
Felisbino MB, Rubino M, Travers JG, Schuetze KB, Lemieux ME, Anseth KS, et al. Substrate stiffness modulates cardiac fibroblast activation, senescence, and proinflammatory secretory phenotype. Am J Physiol Heart Circ Physiol. 2024;326:H61–73.
Toutfaire M, Dumortier E, Fattaccioli A, Van Steenbrugge M, Proby CM, Debacq-Chainiaux F. Unraveling the interplay between senescent dermal fibroblasts and cutaneous squamous cell carcinoma cell lines at different stages of tumorigenesis. Int J Biochem Cell Biol. 2018;98:113–26.
Nishimura J, Morita Y, Tobe-Nishimoto A, Kitahira Y, Takayama S, Kishimoto S, et al. CDDP-induced desmoplasia-like changes in oral cancer tissues are related to SASP-related factors induced by the senescence of cancer cells. Int Immunopharmacol. 2024;136:112377.
Bai J, Wang Y, Wang J, Zhai J, He F, Zhu G. Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling. Am J Physiol Cell Physiol. 2020;318:C1005-17.
Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782–90.
Chaudhuri O, Koshy ST, da Branco Cunha C, Shin JW, Verbeke CS, Allison KH, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970–8.
Gkretsi V, Stylianopoulos T. Cell adhesion and matrix stiffness: coordinating cancer cell invasion and metastasis. Front Oncol. 2018;8:145.
Cirillo N, Vicidomini A, McCullough M, Gambardella A, Hassona Y, Prime SS, et al. A hyaluronic acid-based compound inhibits fibroblast senescence induced by oxidative stress in vitro and prevents oral mucositis in vivo. J Cell Physiol. 2015;230:1421–9.
Yao X, Li H, Chen L, Tan LP. Uv-induced senescence of human dermal fibroblasts restrained by low-stiffness matrix by inhibiting NF-κB activation. Engineered Regeneration. 2022;3:365–73.
Wiley CD, Liu S, Limbad C, Zawadzka AM, Beck J, Demaria M, et al. SILAC Analysis Reveals Increased Secretion of Hemostasis-Related Factors by Senescent Cells. Cell Rep. 2019;28:3329–37 (e3325).
Kim H, Jang J, Song MJ, Park CH, Lee DH, Lee SH, et al. Inhibition of matrix metalloproteinase expression by selective clearing of senescent dermal fibroblasts attenuates ultraviolet-induced photoaging. Biomed Pharmacother. 2022;150:113034.
Gwak H, Hong S, Lee SH, Kim IW, Kim Y, Kim H, et al. Low-intensity pulsed ultrasound treatment selectively stimulates senescent cells to promote SASP factors for immune cell recruitment. Aging Cell. 2025;24:e14486.
Tian YT, Ma LP, Ding CY, Liu MM, Wang SN, Tian M, et al. Autophagy regulates X-ray radiation-induced premature senescence through STAT3-Beclin1-p62 pathway in lung adenocarcinoma cells. Int J Radiat Biol. 2022;98:1432–41.
Huart C, Fransolet M, Demazy C, Le Calvé B, Lucas S, Michiels C, et al. Taking advantage of the senescence-promoting effect of olaparib after X-ray and proton irradiation using the senolytic drug, ABT-263. Cancers (Basel). 202;14(6):1460. https://doi.org/10.3390/cancers14061460.
Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol. 2017;27:2652–60 e2654.
Alessio N, Acar MB, Squillaro T, Aprile D, Ayaz-Guner S, Di Bernardo G, et al. Progression of irradiated mesenchymal stromal cells from early to late senescence: Changes in SASP composition and anti-tumour properties. Cell Prolif. 2023;56:e13401.
Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25(2):214–21.
Magkouta S, Markaki E, Evangelou K, Petty R, Verginis P, Gorgoulis V. Decoding T cell senescence in cancer: is revisiting required? Semin Cancer Biol. 2025;108:33–47.
Ge Z, Wu S, Zhang Z, Ding S. Mechanism of tumor cells escaping from immune surveillance of NK cells. Immunopharmacol Immunotoxicol. 2020;42:187–98.
Lin D, Lei L, Liu Y, Zhang Y, Hu B, Bao G, et al. Membrane IL1alpha inhibits the development of hepatocellular carcinoma via promoting T- and NK-cell activation. Cancer Res. 2016;76:3179–88.
Oka N, Markova T, Tsuzuki K, Li W, El-Darawish Y, Pencheva-Demireva M, et al. IL-12 regulates the expansion, phenotype, and function of murine NK cells activated by IL-15 and IL-18. Cancer Immunol Immunother. 2020;69:1699–712.
Gotthardt D, Trifinopoulos J, Sexl V, Putz EM. JAK/STAT cytokine signaling at the crossroad of NK cell development and maturation. Front Immunol. 2019;10:2590.
Kim J, Kim JS, Lee HK, Kim HS, Park EJ, Choi JE, et al. Cxcr3-deficient natural killer cells fail to migrate to B16F10 melanoma cells. Int Immunopharmacol. 2018;63:66–73.
Benigni G, Dimitrova P, Antonangeli F, Sanseviero E, Milanova V, Blom A, et al. CXCR3/CXCL10 axis regulates neutrophil-NK cell cross-talk determining the severity of experimental osteoarthritis. J Immunol. 2017;198:2115–24.
Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, Ilangumaran S. IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8+ T lymphocytes. J Immunol. 2008;180:7958–68.
Yang Y, Ochando J, Yopp A, Bromberg JS, Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J Immunol. 2005;174:2720–9.
Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9.
Gesser B, Lund M, Lohse N, Vestergaad C, Matsushima K, Sindet-Pedersen S, et al. IL-8 induces T cell chemotaxis, suppresses IL-4, and up-regulates IL-8 production by CD4+ T cells. J Leukoc Biol. 1996;59:407–11.
Zhang H, Yan D, Shi X, Liang H, Pang Y, Qin N, et al. Transmembrane TNF-alpha mediates “forward” and “reverse” signaling, inducing cell death or survival via the NF-kappaB pathway in Raji Burkitt lymphoma cells. J Leukoc Biol. 2008;84:789–97.
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–68 e117.
Haabeth OA, Lorvik KB, Yagita H, Bogen B, Corthay A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology. 2016;5:e1039763.
Lekan AA, Weiner LM. The role of chemokines in orchestrating the immune response to pancreatic ductal adenocarcinoma. Cancers (Basel). 2024;16(3):559. https://doi.org/10.3390/cancers16030559.
Freeman P, Bellomo G, Ireland L, Abudula M, Luckett T, Oberst M, et al. Inhibition of insulin-like growth factors increases production of CXCL9/10 by macrophages and fibroblasts and facilitates CD8(+) cytotoxic T cell recruitment to pancreatic tumours. Front Immunol. 2024;15:1382538.
Gunassekaran GR, PoongkavithaiVadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137.
Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR, et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12:8977–93.
Ren F, Fan M, Mei J, Wu Y, Liu C, Pu Q, et al. Interferon-gamma and celecoxib inhibit lung-tumor growth through modulating M2/M1 macrophage ratio in the tumor microenvironment. Drug Des Devel Ther. 2014;8:1527–38.
Wang Q, Cheng F, Ma TT, Xiong HY, Li ZW, Xie CL, et al. Interleukin-12 inhibits the hepatocellular carcinoma growth by inducing macrophage polarization to the M1-like phenotype through downregulation of Stat-3. Mol Cell Biochem. 2016;415:157–68.
Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–62.
Tekin C, Aberson HL, Bijlsma MF, Spek CA. Early macrophage infiltrates impair pancreatic cancer cell growth by TNF-alpha secretion. BMC Cancer. 2020;20:1183.
Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8.
Dominguez D, Ye C, Geng Z, Chen S, Fan J, Qin L, et al. Exogenous IL-33 restores dendritic cell activation and maturation in established cancer. J Immunol. 2017;198:1365–75.
Liu S, Yu Y, Zhang M, Wang W, Cao X. The involvement of TNF-alpha-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-beta-stimulated human dendritic cells to tumor cells. J Immunol. 2001;166:5407–15.
Li H, Wang C, Yu J, Cao S, Wei F, Zhang W, et al. Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery. Cytotherapy. 2009;11:1076–83.
Yang T, Zhang W, Wang L, Xiao C, Wang L, Gong Y, et al. Co-culture of dendritic cells and cytokine-induced killer cells effectively suppresses liver cancer stem cell growth by inhibiting pathways in the immune system. BMC Cancer. 2018;18:984.
Chaudhry UI, Kingham TP, Plitas G, Katz SC, Raab JR, DeMatteo RP. Combined stimulation with interleukin-18 and CpG induces murine natural killer dendritic cells to produce IFN-gamma and inhibit tumor growth. Cancer Res. 2006;66:10497–504.
Chen Y, Ma L, Cheng Z, Hu Z, Xu Y, Wu J, et al. Senescent fibroblast facilitates re-epithelization and collagen deposition in radiation-induced skin injury through IL-33-mediated macrophage polarization. J Transl Med. 2024;22:176.
Lim HX, Choi S, Cho D, Kim TS. IL-33 inhibits the differentiation and immunosuppressive activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Immunol Cell Biol. 2017;95:99–107.
Choi JN, Sun EG, Cho SH. IL-12 enhances immune response by modulation of myeloid derived suppressor cells in tumor microenvironment. Chonnam Med J. 2019;55(2):31–9.
Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011;133:221–38.
Cao X, Leonard K, Collins LI, Cai SF, Mayer JC, Payton JE, et al. Interleukin 12 stimulates IFN-gamma-mediated inhibition of tumor-induced regulatory T-cell proliferation and enhances tumor clearance. Cancer Res. 2009;69:8700–9.
Tian Y, Yuan C, Ma D, Zhang Y, Liu Y, Zhang W, et al. IL-21 and IL-12 inhibit differentiation of Treg and TH17 cells and enhance cytotoxicity of peripheral blood mononuclear cells in patients with cervical cancer. Int J Gynecol Cancer. 2011;21(2011):1672–8.
Takasugi M, Yoshida Y, Ohtani N. Cellular senescence and the tumour microenvironment. Mol Oncol. 2022;16(2):3333–51.
Gonzalez-Meljem JM, Apps JR, Fraser HC. Paracrine roles of cellular senescence in promoting tumourigenesis. Br J Cancer. 2018;118:1283–8.
Chen C, Chen J, Zhang Y, Zhang Q, Shi H. Senescence-associated secretory phenotype in lung cancer: remodeling the tumor microenvironment for metastasis and immune suppression. Front Oncol. 2025;15:1605085.
Alessio N, Aprile D, Squillaro T, Di Bernardo G, Finicelli M, Melone MA, et al. The senescence-associated secretory phenotype (SASP) from mesenchymal stromal cells impairs growth of immortalized prostate cells but has no effect on metastatic prostatic cancer cells. Aging (Albany NY). 2019;11:5817–28.
Ioannidou A, Goulielmaki E, Garinis GA. DNA damage: from chronic inflammation to age-related deterioration. Front Genet. 2016;7:187.
Alspach E, Flanagan KC, Luo X, Ruhland MK, Huang H, Pazolli E, et al. P38MAPK plays a crucial role in stromal-mediated tumorigenesis. Cancer Discov. 2014;4:716–29.
Kojima H, Inoue T, Kunimoto H, Nakajima K. IL-6-STAT3 signaling and premature senescence. JAKSTAT. 2013;2(2):e25763.
Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402.
Wan D, Jiang W, Hao J. Research advances in how the cGAS-STING pathway controls the cellular inflammatory response. Front Immunol. 2020;11:615.
Kawakami S, Johmura Y, Nakanishi M. Intracellular acidification and glycolysis modulate inflammatory pathway in senescent cells. J Biochem. 2024;176:97–108.
Yasuda T, Baba H, Ishimoto T. Cellular senescence in the tumor microenvironment and context-specific cancer treatment strategies. FEBS J. 2023;290(2023):1290–302.
Bogdanova DA, Kolosova ED, Pukhalskaia TV, Levchuk KA, Demidov ON, Belotserkovskaya EV. The differential effect of senolytics on SASP cytokine secretion and regulation of EMT by CAFs. Int J Mol Sci. 2024;25(7):4031. https://doi.org/10.3390/ijms25074031.
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br J Cancer. 2014;110:724–32.
Saitoh M. Transcriptional regulation of EMT transcription factors in cancer. Semin Cancer Biol. 2023;97:21–9.
Xiang H, Ramil CP, Hai J, Zhang C, Wang H, Watkins AA, et al. Cancer-associated fibroblasts promote immunosuppression by inducing ROS-Generating Monocytic MDSCs in lung squamous cell carcinoma, cancer. Immunol Res. 2020;8:436–50.
Oo MW, Kawai H, Takabatake K, Tomida S, Eguchi T, Ono K, et al. Resident stroma-secreted chemokine CCL2 governs myeloid-derived suppressor cells in the tumor microenvironment. JCI Insight. 2022;7(1):e148960. https://doi.org/10.1172/jci.insight.148960.
Ye J, Baer JM, Faget DV, Morikis VA, Ren Q, Melam A, et al. Senescent CAFs mediate immunosuppression and drive breast cancer progression. Cancer Discov. 2024;14:1302–23.
Assouline B, Kahn R, Hodali L, Condiotti R, Engel Y, Elyada E, et al. Senescent cancer-associated fibroblasts in pancreatic adenocarcinoma restrict CD8(+) T cell activation and limit responsiveness to immunotherapy in mice. Nat Commun. 2024;15:6162.
Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–96.
Lawrenson K, Grun B, Benjamin E, Jacobs IJ, Dafou D, Gayther SA. Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia. 2010;12:317–25.
Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–48.
Huang B, Lang X, Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol. 2022;12:1023177.
Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–62.
Zhao J, Wang Z, Tian Y, Ning J, Ye H. T cell exhaustion and senescence for ovarian cancer immunotherapy. Semin Cancer Biol. 2024;104–105:1–15.
Gao HF, Cheng CS, Tang J, Li Y, Chen H, Meng ZQ, et al. CXCL9 chemokine promotes the progression of human pancreatic adenocarcinoma through STAT3-dependent cytotoxic T lymphocyte suppression. Aging (Albany NY). 2020;12(2):502–17.
Chibaya L, Snyder J, Ruscetti M. Senescence and the tumor-immune landscape: implications for cancer immunotherapy. Semin Cancer Biol. 2022;86:827–45.
Nakagami H. Cellular senescence and senescence-associated T cells as a potential therapeutic target. Geriatr Gerontol Int. 2020;20:97–100.
Du M, Sun L, Guo J, Lv H. Macrophages and tumor-associated macrophages in the senescent microenvironment: from immunosuppressive TME to targeted tumor therapy. Pharmacol Res. 2024;204:107198.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40.
Zhou J, Zheng S, Liu T, Liu Q, Chen Y, Tan D, et al. IL-1beta from M2 macrophages promotes migration and invasion of ESCC cells enhancing epithelial-mesenchymal transition and activating NF-kappaB signaling pathway. J Cell Biochem. 2018;119:7040–52.
Cho U, Kim B, Kim S, Han Y, Song YS. Pro-inflammatory M1 macrophage enhances metastatic potential of ovarian cancer cells through NF-kappaB activation. Mol Carcinog. 2018;57:235–42.
Xu YD, Cheng M, Shang PP, Yang YQ. Role of IL-6 in dendritic cell functions. J Leukoc Biol. 2022;111:695–709.
Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–54.
Shi H, Han X, Sun Y, Shang C, Wei M, Ba X, et al. Chemokine (C-X-C motif) ligand 1 and CXCL2 produced by tumor promote the generation of monocytic myeloid-derived suppressor cells. Cancer Sci. 2018;109:3826–39.
Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6(237):ra267.
Bullock K, Richmond A. Suppressing MDSC recruitment to the tumor microenvironment by antagonizing CXCR2 to enhance the efficacy of immunotherapy. Cancers (Basel). 2021;13(24):6293. https://doi.org/10.3390/cancers13246293.
Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest. 2013;123:4859–74.
Schmitt EG, Haribhai D, Williams JB, Aggarwal P, Jia S, Charbonnier LM, et al. IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy. J Immunol. 2012;189:5638–48.
Davidson TS, Shevach EM. Polyclonal treg cells modulate T effector cell trafficking. Eur J Immunol. 2011;41(2011):2862–70.
Budhu S, Schaer DA, Li Y, Toledo-Crow R, Panageas K, Yang X, Zhong H, Houghton AN, Silverstein SC, Merghoub T, Wolchok JD. Blockade of surface-bound TGF-beta on regulatory T cells abrogates suppression of effector T cell function in the tumor microenvironment. Sci Signal. 2017;10.(494):eaak9702.
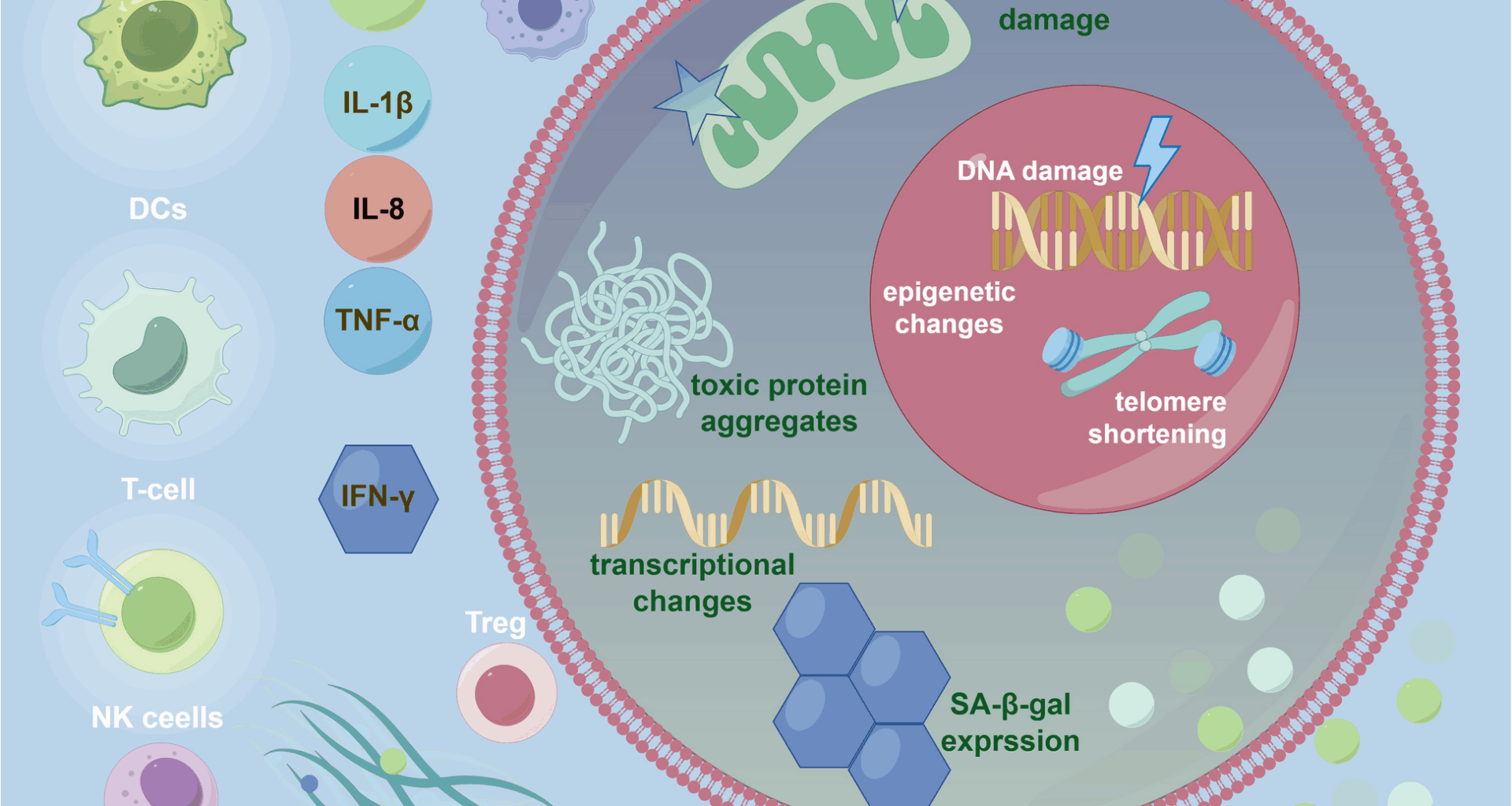
Borodkina AV, Deryabin PI, Giukova AA, Nikolsky NN. “Social Life” of Senescent Cells: What Is SASP and Why Study It? Acta Naturae. 2018;10(2):4–14.
Levi N, Papismadov N, Solomonov I, Sagi I, Krizhanovsky V. The ECM path of senescence in aging: components and modifiers. FEBS J. 2020;287:2636–46.
Higashiguchi M, Murakami H, Akita H, Kobayashi S, Takahama S, Iwagami Y, Yamada D, Tomimaru Y, Noda T, Gotoh K, Doki Y, Yamamoto T, Eguchi H. The impact of cellular senescence and senescence‑associated secretory phenotype in cancer‑associated fibroblasts on the malignancy of pancreatic cancer. Oncol Rep. 2023;49(5):98.
Wang T, Notta F, Navab R, Joseph J, Ibrahimov E, Xu J, et al. Senescent carcinoma-associated fibroblasts upregulate IL8 to enhance prometastatic phenotypes. Mol Cancer Res. 2017;15:3–14.
Yamao T, Yamashita YI, Yamamura K, Nakao Y, Tsukamoto M, Nakagawa S, et al. Cellular senescence, represented by expression of Caveolin-1, in cancer-associated fibroblasts promotes tumor invasion in pancreatic cancer. Ann Surg Oncol. 2019;26:1552–9.
Chowdhury SR, Murphy KC, Parikh CN, DeMarco KD, Zhou L, Ruscetti M. Measuring the impact of therapy-induced senescence on NK cell phenotypes in cancer. Methods Cell Biol. 2024;190:171–201.
Rautela J, Huntington ND. IL-15 signaling in NK cell cancer immunotherapy. Curr Opin Immunol. 2017;44:1–6.
Ma S, Caligiuri MA, Yu J. Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol. 2022;43:833–47.
Admasu TD, Yu JS. Harnessing immune rejuvenation: advances in overcoming T cell senescence and exhaustion in cancer immunotherapy. Aging Cell. 2025;24:e70055.
Nizzoli G, Krietsch J, Weick A, Steinfelder S, Facciotti F, Gruarin P, et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood. 2013;122:932–42.
Mehrotra PT, Wu D, Crim JA, Mostowski HS, Siegel JP. Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol. 1993;151:2444–52.
Thomas DA, Massague J. Tgf-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80.
Sharma S, Stolina M, Lin Y, Gardner B, Miller PW, Kronenberg M, et al. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol. 1999;163:5020–8.
Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–37.
Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–4.
Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7:52294–306.
Zhu L, Fu X, Chen X, Han X, Dong P. M2 macrophages induce EMT through the TGF-beta/Smad2 signaling pathway. Cell Biol Int. 2017;41:960–8.
Ma X, Gao Y, Chen Y, Liu J, Yang C, Bao C, et al. M2-type macrophages induce Tregs generation by activating the TGF-beta/Smad signalling pathway to promote colorectal cancer development. Onco Targets Ther. 2021;14:5391–402.
McBride JM, Jung T, de Vries JE, Aversa G. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell Immunol. 2002;215:162–72.
Weber R, Groth C, Lasser S, Arkhypov I, Petrova V, Altevogt P, et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021;359:104254.
Weber R, Riester Z, Hüser L, Sticht C, Siebenmorgen A, Groth C, et al. IL-6 regulates CCR5 expression and immunosuppressive capacity of MDSC in murine melanoma. J Immunother Cancer. 2020;8(2):e000949. https://doi.org/10.1136/jitc-2020-000949.
Tobin RP, Jordan KR, Kapoor P, Spongberg E, Davis D, Vorwald VM, et al. IL-6 and IL-8 are linked with myeloid-derived suppressor cell accumulation and correlate with poor clinical outcomes in melanoma patients. Front Oncol. 2019;9:1223.
Lee CS, Baek J, Han SY. The role of kinase modulators in cellular senescence for use in cancer treatment. Molecules. 2017;22(9):1411. https://doi.org/10.3390/molecules22091411.
Peng S, Sen B, Mazumdar T, Byers LA, Diao L, Wang J, et al. Dasatinib induces DNA damage and activates DNA repair pathways leading to senescence in non-small cell lung cancer cell lines with kinase-inactivating BRAF mutations. Oncotarget. 2016;7:565–79.
Rahimifard M, Baeeri M, Bahadar H, Moini-Nodeh S, Khalid M, Haghi-Aminjan H, Mohammadian H, Abdollahi M. Molecular and biochemical evidence of edaravone’s impact on Dasatinib-induced AGS cell senescence: a promising strategy for gastric cancer therapy. Curr Med Chem. 2025. Feb 24 (online ahead of print). https://doi.org/10.2174/0109298673338322250211111422.
Ozsoy S, Becer E, Kabadayi H, Vatansever HS, Yucecan S. Quercetin-mediated apoptosis and cellular senescence in human colon cancer. Anticancer Agents Med Chem. 2020;20:1387–96.
Wang L, Xiong B, Lu W, Cheng Y, Zhu J, Ai G, et al. Senolytic drugs dasatinib and quercetin combined with carboplatin or olaparib reduced the peritoneal and adipose tissue metastasis of ovarian cancer. Biomed Pharmacother. 2024;174:116474.
Islam MT, Tuday E, Allen S, Kim J, Trott DW, Holland WL, et al. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell. 2023;22:e13767.
Novais EJ, Tran VA, Johnston SN, Darris KR, Roupas AJ, Sessions GA, et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12:5213.
Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28.
Rzeszutek I, Cybularczyk-Cecotka M, Deregowska A, Stec P, Wnuk M, Kolodziej O, et al. New mitochondria-targeted fisetin derivative compromises mitophagy and limits survival of drug-induced senescent breast cancer cells. J Med Chem. 2024;67:17676–89.
Mullen M, Nelson AL, Goff A, Billings J, Kloser H, Huard C, et al. Fisetin attenuates cellular senescence accumulation during culture expansion of human adipose-derived stem cells. Stem Cells. 2023;41:698–710.
Mahoney SA, Venkatasubramanian R, Darrah MA, Ludwig KR, VanDongen NS, Greenberg NT, et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. Aging Cell. 2024;23:e14060.
Jochems F, Baltira C, MacDonald JA, Daniels V, Mathur A, de Gooijer MC, et al. Senolysis by ABT-263 is associated with inherent apoptotic dependence of cancer cells derived from the non-senescent state. Cell Death Differ. 2025;32:855–65.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83.
Zhu RZ, Li BS, Gao SS, Seo JH, Choi BM. Luteolin inhibits H2O2-induced cellular senescence via modulation of SIRT1 and p53. Korean J Physiol Pharmacol. 2021;25:297–305.
Yan Y, Huang H, Su T, Huang W, Wu X, Chen X, et al. Luteolin mitigates photoaging caused by UVA-induced fibroblast senescence by modulating oxidative stress pathways. Int J Mol Sci. 2025;26(5):1809. https://doi.org/10.3390/ijms26051809.
Sun Y, Hu X, Hu G, Xu C, Jiang H. Curcumin attenuates hydrogen peroxide-induced premature senescence via the activation of SIRT1 in human umbilical vein endothelial cells. Biol Pharm Bull. 2015;38(2):1134–41.
Jin H, Lian N, Zhang F, Chen L, Chen Q, Lu C, et al. Activation of PPARgamma/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell Death Dis. 2016;7:e2189.
Grabowska W, Mosieniak G, Achtabowska N, Czochara R, Litwinienko G, Bojko A, et al. Curcumin induces multiple signaling pathways leading to vascular smooth muscle cell senescence. Biogerontology. 2019;20:783–98.
Li W, He Y, Zhang R, Zheng G, Zhou D. The curcumin analog EF24 is a novel senolytic agent. Aging (Albany NY). 2019;11(2):771–82.
Zhang Y, Liu J, Zheng R, Hou K, Zhang Y, Jia T, et al. Curcumin analogue EF24 prevents alveolar epithelial cell senescence to ameliorate idiopathic pulmonary fibrosis via activation of PTEN. Phytomedicine. 2024;133:155882.
Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8:422.
Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD. Hsp90 inhibitors as senolytic drugs to extend healthy aging. Cell Cycle. 2018;17:1048–55.
Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY). 2016;8:2915–26.
Kapoor N, Bhattacharjee A, Chakraborty S, Katti DS. Piperlongumine mediates amelioration of osteoarthritis via inhibition of chondrocyte senescence and inflammation in a goat ex vivo model. Eur J Pharmacol. 2023;961:176136.
Bode-Boger SM, Martens-Lobenhoffer J, Tager M, Schroder H, Scalera F. Aspirin reduces endothelial cell senescence. Biochem Biophys Res Commun. 2005;334:1226–32.
Feng M, Kim J, Field K, Reid C, Chatzistamou I, Shim M. Aspirin ameliorates the long-term adverse effects of doxorubicin through suppression of cellular senescence. FASEB Bioadv. 2019;1:579–90.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–35.
Mohamad Anuar NN, Nor Hisam NS, Liew SL, Ugusman A. Clinical review: Navitoclax as a pro-apoptotic and anti-fibrotic agent. Front Pharmacol. 2020;11:564108.
Wong M, Tan N, Zha J, Peale FV, Yue P, Fairbrother WJ, et al. Navitoclax (ABT-263) reduces Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol Cancer Ther. 2012;11:1026–35.
Nor Hisam NS, Ugusman A, Rajab NF, Ahmad MF, Fenech M, Liew SL, et al. Combination therapy of navitoclax with chemotherapeutic agents in solid tumors and blood cancer: a review of current evidence. Pharmaceutics. 2021;13(9):1353. https://doi.org/10.3390/pharmaceutics13091353.
Angelopoulou A, Theocharous G, Valakos D, Polyzou A, Magkouta S, Myrianthopoulos V, et al. Loss of the tumour suppressor LKB1/STK11 uncovers a leptin-mediated sensitivity mechanism to mitochondrial uncouplers for targeted cancer therapy. Mol Cancer. 2024;23:147.
Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, et al. Quercetin: a pleiotropic kinase inhibitor against cancer. Cancer Treat Res. 2014;159:185–205.
Russo GL, Russo M, Spagnuolo C. The pleiotropic flavonoid quercetin: from its metabolism to the inhibition of protein kinases in chronic lymphocytic leukemia. Food Funct. 2014;5:2393–401.
Imran M, Saeed F, Gilani SA, Shariati MA, Imran A, Afzaal M, et al. Fisetin: an anticancer perspective. Food Sci Nutr. 2021;9:3–16.
Rahmani AH, Almatroudi A, Allemailem KS, Khan AA, Almatroodi SA. The potential role of fisetin, a flavonoid in cancer prevention and treatment. Molecules. 2022;27(24):9009. https://doi.org/10.3390/molecules27249009.
Samaraweera L, Adomako A, Rodriguez-Gabin A, McDaid HM. A novel indication for panobinostat as a senolytic drug in NSCLC and HNSCC. Sci Rep. 2017;7:1900.
Ibrahim N, Buchbinder EI, Granter SR, Rodig SJ, Giobbie-Hurder A, Becerra C, et al. A phase I trial of panobinostat (LBH589) in patients with metastatic melanoma. Cancer Med. 2016;5:3041–50.
Wakita M, Takahashi A, Sano O, Loo TM, Imai Y, Narukawa M, et al. A bet family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nat Commun. 2020;11:1935.
Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–59.
Yu L, Liu J, Huang X, Jiang Q. Adverse effects of dasatinib on glucose-lipid metabolism in patients with chronic myeloid leukaemia in the chronic phase. Sci Rep. 2019;9:17601.
Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–16.
Muñoz-Espín D, Rovira M, Galiana I, Giménez C, Lozano-Torres B, Paez-Ribes M, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med. 2018;10(9):e9355. https://doi.org/10.15252/emmm.201809355.
Magkouta S, Veroutis D, Papaspyropoulos A, Georgiou M, Lougiakis N, Pippa N, et al. Generation of a selective senolytic platform using a micelle-encapsulated Sudan Black B conjugated analog. Nat Aging. 2025;5:162–75.
Admasu TD, Kim K, Rae M, Avelar R, Gonciarz RL, Rebbaa A, et al. Selective ablation of primary and paracrine senescent cells by targeting iron dyshomeostasis. Cell Rep. 2023;42:112058.
Wang R, Sunchu B, Perez VI. Rapamycin and the inhibition of the secretory phenotype. Exp Gerontol. 2017;94:89–92.
Turgut S, Atasever E, Cebe T, Andican G, Cakatay U. Senotherapeutic repurposing of metformin for age-related diseases and their signaling pathways. Mol Biol Rep. 2025;52:410.
Hu Q, Peng J, Jiang L, Li W, Su Q, Zhang J, et al. Metformin as a senostatic drug enhances the anticancer efficacy of CDK4/6 inhibitor in head and neck squamous cell carcinoma. Cell Death Dis. 2020;11:925.
Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain MA, et al. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med. 2016;8:362ra144.
Pemmaraju N, Garcia JS, Potluri J, Harb JG, Sun Y, Jung P, et al. Addition of navitoclax to ongoing ruxolitinib treatment in patients with myelofibrosis (REFINE): a post-hoc analysis of molecular biomarkers in a phase 2 study. Lancet Haematol. 2022;9:e434–44.
Ge H, Ke J, Xu N, Li H, Gong J, Li X, et al. Dexamethasone alleviates pemetrexed-induced senescence in non-small-cell lung cancer. Food Chem Toxicol. 2018;119:86–97.
Jiang H, Wang GT, Wang Z, Ma QY, Ma ZH. Resveratrol inhibits pancreatic cancer proliferation and metastasis by depleting senescent tumor-associated fibroblasts. World J Gastrointest Oncol. 2024;16(2024):3980–93.
Luan FL, Ding R, Sharma VK, Chon WJ, Lagman M, Suthanthiran M. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003;63:917–26.
Jin P, Li X, Xia Y, Li H, Li X, Yang ZY, et al. Bepotastine sensitizes ovarian cancer to PARP inhibitors through suppressing NF-kappaB-triggered SASP in cancer-associated fibroblasts. Mol Cancer Ther. 2023;22:447–58.
Beppu M, Ikebe T, Shirasuna K. The inhibitory effects of immunosuppressive factors, dexamethasone and interleukin-4, on NF-kappaB-mediated protease production by oral cancer. Biochim Biophys Acta. 2002;1586:11–22.
Al-Mansour F, Alraddadi A, He B, Saleh A, Poblocka M, Alzahrani W, et al. Characterization of the HDAC/PI3K inhibitor CUDC-907 as a novel senolytic. Aging (Albany NY). 2023;15:2373–94.
Feldmeyer L, Hofbauer GF, Boni T, French LE, Hafner J. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422–4.
Nguyen VN, Abagyan R, Tsunoda SM. Mtor inhibitors associated with higher cardiovascular adverse events-a large population database analysis. Clin Transplant. 2021;35:e14228.
Giles AJ, Hutchinson MND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6:51.
Yokoyama T, Kohn EC, Brill E, Lee JM. Apoptosis is augmented in high-grade serous ovarian cancer by the combined inhibition of Bcl-2/Bcl-xL and PARP. Int J Oncol. 2017;50:1064–74.
Wang T, Liu W, Shen Q, Tao R, Li C, Shen Q, et al. Combination of PARP inhibitor and CDK4/6 inhibitor modulates cGAS/STING-dependent therapy-induced senescence and provides “one-two punch” opportunity with anti-PD-L1 therapy in colorectal cancer. Cancer Sci. 2023;114:4184–201.
Squarzoni S, Schena E, Sabatelli P, Mattioli E, Capanni C, Cenni V, et al. Interleukin-6 neutralization ameliorates symptoms in prematurely aged mice. Aging Cell. 2021;20:e13285.
Su H, Lin X, Paredong A, Yao C, Zhang Y, Geng M, et al. IL-6/GATA2/SERPINE1 pathway is implicated in regulating cellular senescence after acute kidney injury. Front Mol Biosci. 2025;12:1538526.
Xiong J, Dong L, Lv Q, Yin Y, Zhao J, Ke Y, et al. Targeting senescence-associated secretory phenotypes to remodel the tumour microenvironment and modulate tumour outcomes. Clin Transl Med. 2024;14:e1772.
Al-Jomah N, Al-Mohanna FH, Aboussekhra A. Tocilizumab suppresses the pro-carcinogenic effects of breast cancer-associated fibroblasts through inhibition of the STAT3/AUF1 pathway. Carcinogenesis. 2021;42:1439–48.
Blay JY, Brahmi M, Dufresne A, Swalduz A, Avrillon V, Assaad S, et al. Anti-IL-6R ab tocilizumab to treat paraneoplastic inflammatory syndrome of solid cancers. ESMO Open. 2025;10(1):104088.
Guo Y, Nemeth J, O’Brien C, Susa M, Liu X, Zhang Z, et al. Effects of siltuximab on the IL-6-induced signaling pathway in ovarian cancer. Clin Cancer Res. 2010;16:5759–69.
Najjary S, Mohammadzadeh R, Mokhtarzadeh A, Mohammadi A, Kojabad AB, Baradaran B. Role of miR-21 as an authentic oncogene in mediating drug resistance in breast cancer. Gene. 2020;738:144453.
Nguyen HT, Kacimi SEO, Nguyen TL, Suman KH, Lemus-Martin R, Saleem H, et al. Mir-21 in the cancers of the digestive system and its potential role as a diagnostic, predictive, and therapeutic biomarker. Biology. 2021;10(5):417. https://doi.org/10.3390/biology10050417.
Chen JC, Hsieh YY, Lo HL, Li A, Chou CJ, Yang PM. In vitro and in silico mechanistic insights into miR-21–5p-mediated topoisomerase drug resistance in human colorectal cancer cells. Biomolecules. 2019;9(9):467. https://doi.org/10.3390/biom9090467.
Wang T, Wu J, Liu X, Li S. Serum miR-34a is a potential diagnostic and prognostic marker for osteosarcoma. Int J Clin Exp Pathol. 2017;10:9683–9.
Lian H, Zhou Y, Sun Z, Liu K. Microrna34a is associated with chemotherapy resistance, metastasis, recurrence, survival, and prognosis in patient with osteosarcoma. Medicine (Baltimore). 2022;101:e30722.
Yang S, Li A, Lv L, Zheng Z, Liu P, Min J, et al. Exosomal miRNA-146a-5p derived from senescent hepatocellular carcinoma cells promotes aging and inhibits aerobic glycolysis in liver cells via targeting IRF7. J Cancer. 2024;15:4448–66.
Iguchi T, Nambara S, Masuda T, Komatsu H, Ueda M, Kidogami S, et al. Mir-146a polymorphism (rs2910164) predicts colorectal cancer patients’ susceptibility to liver metastasis. PLoS One. 2016;11:e0165912.
Sun X, Thorne RF, Zhang XD, He M, Li J, Feng S, et al. LncRNA GUARDIN suppresses cellular senescence through a LRP130-PGC1alpha-FOXO4-p21-dependent signaling axis. EMBO Rep. 2020;21:e48796.
Yang W, Du WW, Li X, Yee AJ, Yang BB. Foxo3 activity promoted by non-coding effects of circular RNA and Foxo3 pseudogene in the inhibition of tumor growth and angiogenesis. Oncogene. 2016;35:3919–31.
Panda AC, Grammatikakis I, Kim KM, De S, Martindale JL, Munk R, et al. Identification of senescence-associated circular RNAs (SAC-RNAs) reveals senescence suppressor CircPVT1. Nucleic Acids Res. 2017;45:4021–35.
Min X, Cai MY, Shao T, Xu ZY, Liao Z, Liu DL, et al. A circular intronic RNA ciPVT1 delays endothelial cell senescence by regulating the miR-24-3p/CDK4/pRb axis. Aging Cell. 2022;21:e13529.
Li MF, Zhang DH, Wang LS, Yue CF, Pang LJ, Guo YM, et al. Construction and validation of a SASP-related prognostic signature in patients with acute myeloid leukaemia. J Cell Mol Med. 2024;28:e70017.
Bhatiya M, Pathak S, Banerjee A. Oxidative stress and cellular senescence: the key tumor-promoting factors in colon cancer and beneficial effects of polyphenols in colon cancer prevention. Curr Cancer Ther Rev. 2021;17:292–303.