Data source
This study utilized data from the Korean NHIS, which operates a mandatory universal healthcare system covering over 97% of the Korean population. The NHIS database contains comprehensive longitudinal information on demographic characteristics, insurance eligibility, healthcare utilization, diagnoses coded according to the International Classification of Diseases, 10th Revision (ICD-10), procedures, prescription records, and mortality data from 2002 [22]. In addition, the NHIS provides a standardized health screening program conducted every 1 to 2 years to all insured adults aged ≥ 40 years and to employed individuals aged ≥ 20 years. The program collects data on anthropometric measures, blood pressure, laboratory tests, and health-related behaviors, including smoking, alcohol consumption, and physical activity.
For this study we used the National Sample Cohort, a population-based cohort established by the Korean NHIS [23]. The cohort consists of a 2% representative sample (approximately one million individuals) of the Korean population in 2002, selected through stratified random sampling by age, sex, region, and insurance type. All enrollees have been followed annually through claims, eligibility, and death records until 2019. This study was reviewed by the Institutional Review Board of Seoul National University Bundang Hospital (IRB number X-2018-705-901), in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was officially waived by the IRB, as the study used fully anonymized retrospective data from the NHIS in accordance with national regulations.
Study population and cohort selection
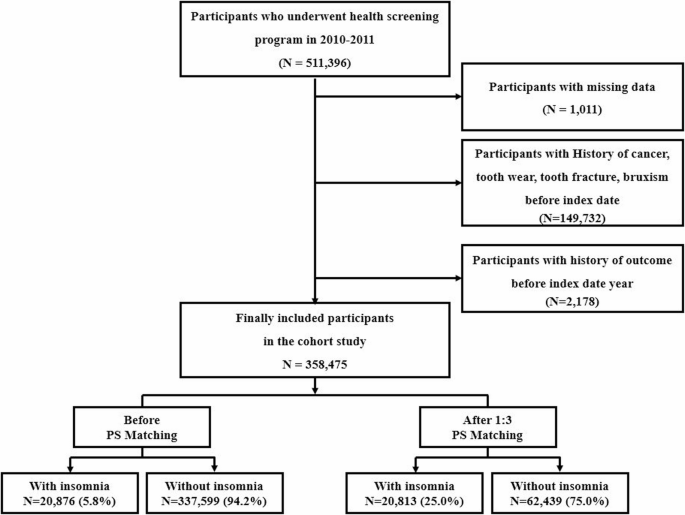
We initially identified 511,396 adults aged ≥ 20 years who participated in AT least one national health screening examination between January 1, 2011, and December 31, 2014. The index date was defined as the most recent health screening for each individual. To identify newly diagnosed cases of insomnia and subsequent TMD, we applied the following exclusion criteria: (1) individuals diagnosed with malignant neoplasms (ICD-10 codes C00–C97) from 2002 to 2014; (2) individuals with prior diagnoses of bruxism (F51.8, F45.8), tooth wear (K03.00–K03.19), or tooth fracture (S02.52–S02.59) before the index date; and (3) individuals diagnosed with either insomnia (F51.0 or G47.0) or TMD (K07.6) prior to or during the index year, which was considered the washout period. After exclusions, a total of 358,475 participants remained for the final analysis.
The insomnia group was defined as individuals with at least one medical claim containing the ICD-10 code G47.0 (disorders of initiating and maintaining sleep) or F51.0 (nonorganic insomnia), recorded as either a principal or additional diagnosis. To enhance diagnostic specificity, individuals were additionally required to have a prescription for hypnotic medications lasting more than seven consecutive days. These criteria were based on prior Korean studies investigating the prevalence and incidence of insomnia [24].
According to the Korean Clinical Practice Guideline for insomnia, 19 pharmacologic treatments are introduced, of which 12 are approved by the Korean Ministry of Food and Drug Safety [25]. To reduce the risk of misclassification, we excluded medications commonly used for other indications such as anxiety, delirium, pain, or depression. In particular, some agents (e.g., antihistamines) listed in the clinical guideline were excluded because they are widely prescribed for non-insomnia indications, such as allergic conditions. Additionally, other agents (e.g., melatonin, eszopiclone) were not yet approved or commercially available in Korea during the cohort inclusion period. As a result, only six hypnotic agents—doxepin, flunitrazepam, flurazepam, triazolam, zolpidem, and trazodone—were included in the analysis [24]. The control group comprised individuals with no recorded diagnosis of insomnia and no prescription history of hypnotics throughout the study period. As of December 31, 2019, 20,876 participants met the criteria for the insomnia group, and 337,599 were assigned to the control group.
To construct a matched cohort, 1:3 PSM was performed. Individuals in the insomnia group were matched to three controls using a greedy nearest-neighbor algorithm with a caliper of 0.2 Matching was based on key baseline covariates, including age, sex, household income, body mass index (BMI), smoking status, and alcohol consumption. After matching, the final cohort comprised 20,813 individuals with insomnia and 62,439 matched controls who were followed for the development of TMD (Fig. 1).
Flowchart of the study population selection
Variable definition and primary outcome
Baseline characteristics, including age, sex, household income, BMI, smoking habits, and alcohol consumption, were assessed as of the index date. Comorbidities were identified using ICD-10 codes recorded within one year prior to the index date. The comorbid conditions included: hypertension (I10–I15), hyperthyroidism (E05.8, E05.9), hypothyroidism (E03.9), diabetes mellitus (E10, E11.8, E11.9, E13, E14.9), dyslipidemia (E78), myocardial infarction (I21, I22, I25.2), stroke (I60–I63), anemia (D46, D50–D53, D55–D64, D74), asthma (J45.0–J45.9), pneumonia (J12–J18), and chronic obstructive pulmonary disease (COPD; J43.1, J43.2, J43.8, J43.9, J44).
The primary outcome was the incidence of TMD, defined as the first occurrence of an outpatient visit with a diagnosis code of K07.6 after the index date, accompanied by a procedure code corresponding to an analytical assessment of TMD (electronic data interchange code: E9040). Participants were followed from the index date until the occurrence of TMD, death, or December 31, 2019, whichever came first. This definition demonstrated high diagnostic performance, with a sensitivity of 95.8%, specificity of 92.3%, positive predictive value of 92.0%, negative predictive value of 96.0%, and overall accuracy of 94.0% (see Supplementary Methods for details).
Statistical analyses
We examined baseline characteristics by applying the Student’s t-test for continuous variables and the chi-square test for categorical variables to compare the insomnia and control groups. The incidence rate of TMD was estimated as the number of newly diagnosed cases divided by the total person-years (PY) of follow-up. Differences in incidence rates between groups were assessed using the normal approximation test for binomial proportions.
To examine the association between insomnia and the risk of developing TMD, we applied Cox proportional hazards regression models to calculate hazard ratios (HRs) with 95% confidence intervals (CIs). Both unadjusted and multivariable-adjusted models were constructed. Covariates in the adjusted model included age, sex, household income, BMI, smoking status, alcohol consumption, and comorbidities such as hypertension, hyperthyroidism, hypothyroidism, diabetes mellitus, myocardial infarction, stroke, anemia, asthma, pneumonia, and COPD.
To address potential confounding, 1:3 PSM was implemented using a nearest-neighbor algorithm without replacement and with a caliper width 0.2. Covariate balance before and after matching was assessed using standardized mean differences (SMDs), with an SMD < 0.1 considered indicative of adequate balance. The Cox regression analysis was applied to the matched sample to ensure consistency and robustness of the findings.
Kaplan–Meier curves were generated to visualize the cumulative incidence of TMD according to insomnia status. Group differences in survival were tested using the log-rank test. Statistical significance was defined as a two-sided p-values less than 0.05. All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
We confirmed the proportional hazards assumption for the Cox regression model using Schoenfeld residuals for all covariates, including insomnia. The global test showed no significant violations, and no time-varying effects were observed. The estimated HRs were stable throughout the follow-up period.