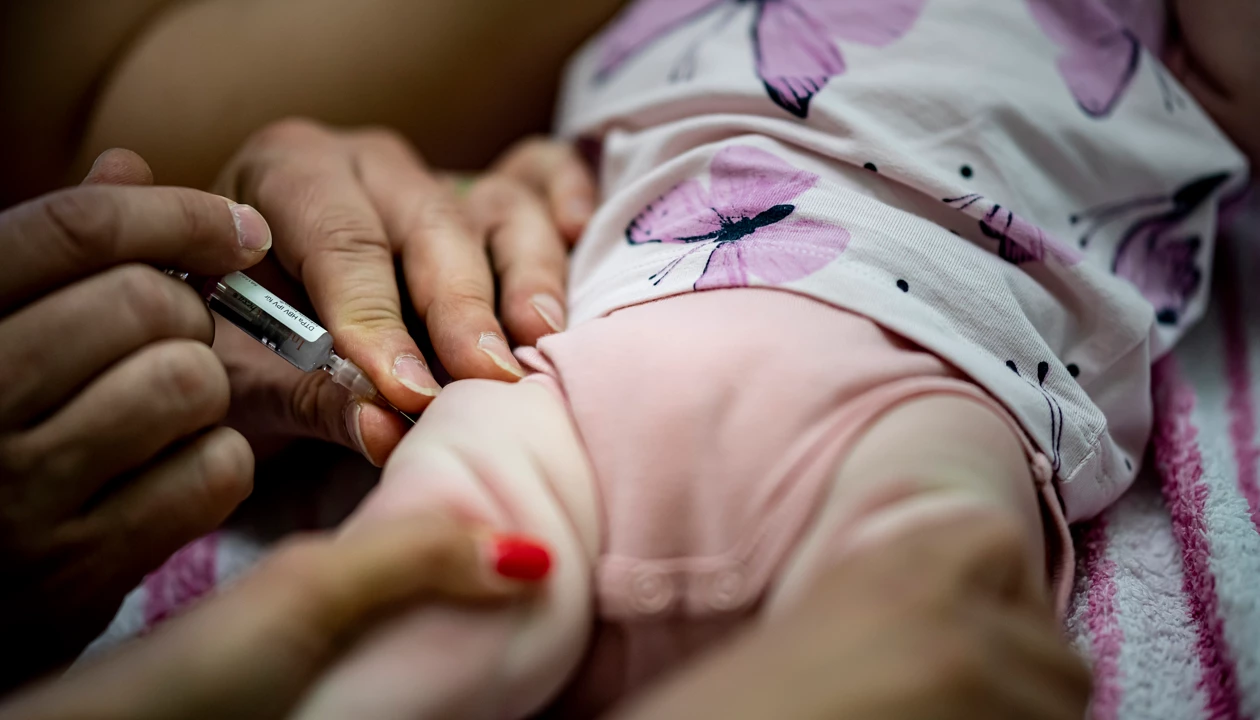
Key Insights
The US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) has delayed a vote on changes to recommendations on giving a hepatitis B vaccine to newborns.
ACIP is expected to make changes that no longer recommend the hepatitis B vaccine be given to a child within 24 h of birth in most cases, despite little evidence that the birth dose vaccine is unsafe.
Experts reject the proposed changes, saying they are unscientifically based and that the CDC can no longer be trusted as a source of vaccine information.
The US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) appears poised to make changes to recommendations surrounding a hepatitis B vaccine given at birth, following a day of contentious discussion.
The group will formally vote on Friday, Dec. 5. The vote was postponed by a day following much confusion over the voting language, which was not made public until hours before the vote was first set.
Vaccine experts expect ACIP will no longer explicitly recommend that all newborns be given a dose of hepatitis B vaccine within 24 h of birth, as the CDC has recommended since 1991. Instead, it is likely that ACIP will recommend that the vaccine be given to children no earlier than 2 months of age, despite little evidence that a delay in vaccination improves health outcomes, unless the birth parent tests positive for hepatitis B antigens.
The change would be the latest in a string of adjustments to the CDC’s vaccine schedule. At the ACIP meeting on Sept. 18 and 19, the panel voted to make changes to guidance for measles, mumps, rubella, and varicella (MMRV) and COVID-19 vaccines. The panel also discussed the hepatitis B birth dose vaccine in September but delayed its vote until December after ACIP members appeared confused about the voting language.
ACIP meetings have been rife with confusion and procedural issues since US Department of Health and Human Services (HHS) secretary Robert F. Kennedy Jr. fired all previous members of the committee in June and replaced them with his own handpicked panel of individuals over the next few months. The reorganization of ACIP is just one of the unilateral moves that Kennedy has made that have cast doubt on vaccine safety data that have repeatedly shown vaccines to be safe and effective.
Paul A. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, has frequently criticized Kennedy’s decisions. “I think we shouldn’t call it an ACIP anymore,” he tells C&EN. “It’s not the ACIP. It’s a group of people like RFK Jr. who have an anti-vaccine, anti-science bias.”
Experts sound alarm over vaccine misinformation
Infectious disease researchers and physicians almost universally reject the newly proposed changes to the vaccine schedule and say they are unscientifically based.
“Many of us in the field of pediatrics, infectious diseases, hepatology, or general pediatrics, or even in public health, really are concerned that missing that first birth dose could really upset a very remarkable and remarkably successful vaccination program that has essentially saved thousands of lives,” says Yvonne Maldonado, a professor of global health and infectious diseases at Stanford University School of Medicine.
Hepatitis B is an incredibly infectious disease, and the birth dose vaccine was established to help fill in the gaps left by hepatitis B screening in the US. Pregnant individuals are typically given a hepatitis B antigen test to detect infection in the first trimester of pregnancy. But some of those tests result in false negatives, and if an infection occurs after the initial screening, it puts the baby at high risk. Children are also susceptible to infection through casual contact with infected individuals if the child is unvaccinated.
The hepatitis B birth dose vaccine, which is given to a child within 24 h of being born, has been a successful initiative, according to published data. Following implementation of the birth dose in 1991, the number of annual hepatitis B infections in children under 1 year old declined from just under 10,000 children to fewer than 20 as of 2023, according to a recent comprehensive review (PDF) published by the University of Minnesota Center for Infectious Disease Research and Policy (CIDRAP). The previous recommendation to give children the birth dose vaccine was not a requirement—parents can choose not to vaccinate their newborn and a minority of them do, according to data presented at the ACIP meeting.
In anticipation of the ACIP vote on the hepatitis B birth dose, the medical community made efforts to debunk the scientific claims behind the change in advance. A new research paper, published as a preprint just days before the ACIP meeting, examined what the cost of moving away from the birth dose in the US would be. The researchers from academic institutions including the Oregon Health and Science University School of Public Health estimated that a change would lead to an additional 1,400 entirely preventable hepatitis B infections in children per year (medRxiv 2025, DOI: 10.1101/2025.11.24.25340907).
ACIP didn’t know what it was voting on before the meeting
While the birth dose was scheduled for discussion and vote on the first day of the ACIP meeting, an overall lack of transparency ultimately led to the delayed vote. Multiple changes to the meeting agenda and voting language were made in the final hours leading up to the meeting, with ACIP members seeing the changes they would be voting on for the first time during the afternoon session.
“We really need to know what we’re voting on,” said Joseph R. Hibbeln, a neuroscientist and voting member of ACIP, at one point late in the meeting’s agenda. “Perhaps this was written by the department of redundancy department,” he remarked later while criticizing the newly available voting language. Much of the confusion was due to last minute changes to the voting language, which had different recommendations based on the results of a hepatitis B antigen test status given during pregnancy.
At the meeting, several notable vaccine critics who have obtained their roles advising the CDC under Kennedy gave presentations casting doubt on the safety and need of a hepatitis B birth dose vaccine.
Cynthia Nevison, a climate scientist who publicly promotes a link between vaccines and autism, presented data suggesting that there was no link between adoption of the universal birth dose and declining hepatitis B infection rates. She also suggested that horizontal transmission of hepatitis B to children in the US is primarily among immigrants with children born outside the US.
Mark Blaxill, who recently joined the CDC as a senior adviser and has published on the link between vaccines and autism—including a paper that was retracted for methodological flaws (J. Autism Dev. Disord. 2023, DOI: 10.1007/s10803-023-06016-4)—presented data suggesting that the hepatitis B birth dose vaccine increases the risk of injury to newborns. But the CIDRAP report refutes those claims and instead suggests the vaccine is safe and that there is no benefit to delaying the first dose of vaccine to 2 months after birth.
Cody Meissner, a professor of pediatrics at the Geisel School of Medicine at Dartmouth College and ACIP voting member, criticized changes made during the last ACIP meeting and was again critical of the presentations made during this meeting. “The way I look at a neonatal birth dose is that it is a safety net, and it’s really for chronically infected mothers who, for one reason or another, do not get tested,” he said.
The hepatitis B vaccine has been in the crosshairs of the anti-vaccine movement for years, according to Elizabeth Jacobs, an epidemiologist and founding member of the public health advocacy organization Defend Public Health. That’s in part because those who are most at risk for the disease are heavily stigmatized, she says. “Hepatitis B can be more common in sex workers or intravenous drug users, and it’s almost like that stigma has pushed people away from [the vaccine],” Jacobs says.
Those who would remove the birth dose from the hepatitis B vaccine schedule also point to a contrast between the US and other high-income countries like Finland and Denmark, which do not vaccinate against hepatitis B in the first hours after birth.
ACIP member Vicky Pebsworth, a nurse who has claimed that vaccines caused her son’s autism, said in the meeting that “the US is an outlier. We are vaccinating infants that no other extremely low-endemicity country vaccinates.”
But Finland and Denmark have social safety nets that the US does not, according to multiple experts. It’s “very disingenuous to make comparisons between the United States and other countries of similar wealth because we do not have nationalized healthcare,” Jacobs says. Getting rid of the hepatitis B birth dose vaccine raises the risk of some children never getting the vaccine because they don’t have access to regular healthcare, according to Offit, Jacobs, and Maldonado.
The American Academy of Pediatrics (AAP), which previously had an advisory role to ACIP but relinquished that role after Secretary Kennedy reconstituted the committee, has continuously published its own recommended vaccine schedule since the late 1930s, well before the formation of ACIP. The AAP did not make the same changes to its recommended vaccine schedule that ACIP did following the September meeting and is not expected to make changes to its recommendations for the hepatitis B birth dose vaccine. It is likely that many pediatricians will still refer to the AAP schedule as their primary source of vaccine guidance.
“I would follow the American Academy of Pediatrics advice, as well as the Vaccine Integrity Project and other groups that are coming out. The one group I wouldn’t trust is ACIP,” Jacobs says.
Max Barnhart is an assistant editor and life sciences reporter at C&EN.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society