We analyzed data from the GLORIA-AF Registry, an international, prospective, multicentre registry programme structured in 3 phases, designed to assess the real-world long-term efficacy and safety of dabigatran etexilate in patients with a recent diagnosed AF. Full details on the study design, study procedures and primary results of GLORIA-AF registry were already reported elsewhere [11,12,13,14]. For this analysis, we considered only the phase III of the registry, which enrolled adult patients (age ≥ 18) with a recent diagnosis of non-valvular AF (i.e. within 3 months, or within 4.5 months in Latin America), and a CHA2DS2-VASc score ≥ 1 between 2014 and 2016. Main exclusion criteria were AF due to a reversible cause, presence of a mechanical heart valve (or patients expected to undergo valve replacement), previous treatment with vitamin K antagonist (VKA) for > 60 days during their lifetime, other clinical indications for OAC, or a limited life expectancy (< 1 year). Approval of the study protocol was provided by local institutional review boards at each participating centre, and written informed consent was obtained from all patients. The study was conducted according to the Declaration of Helsinki and the Good Clinical Practice.
For the purposes of this analysis, we included only patients with complete data on i) the conditions defining each CKM domain and ii) the primary composite outcome (see below).
Definition of the CKM syndrome and its component
To define components of the CKM syndrome, we considered data on clinical characteristics and comorbidities as collected at baseline visit by the study investigators, according to the definition used in the standardized case report forms. We considered the following “domains” of CKM syndrome, along with their qualifying criteria:
Cardiovascular domain: presence of coronary artery disease (CAD), congestive heart failure (CHF; irrespective of ejection fraction), peripheral artery disease (PAD), or a history of stroke/transient ischemic attack (TIA);
Kidney domain: a Creatinine Clearance (CrCl) < 60 ml/min, as calculated by the Cockroft-Gault formula;
Metabolic domain: a body mass index (BMI) ≥ 25 kg/m2 (i.e., overweight or obesity status), diabetes mellitus, or hyperlipidemia.
Any patient presenting with at least one of the conditions or diseases listed above was considered as having that domain (i.e., component) of the CKM syndrome.
For subsequent analyses, we also considered the following variables:
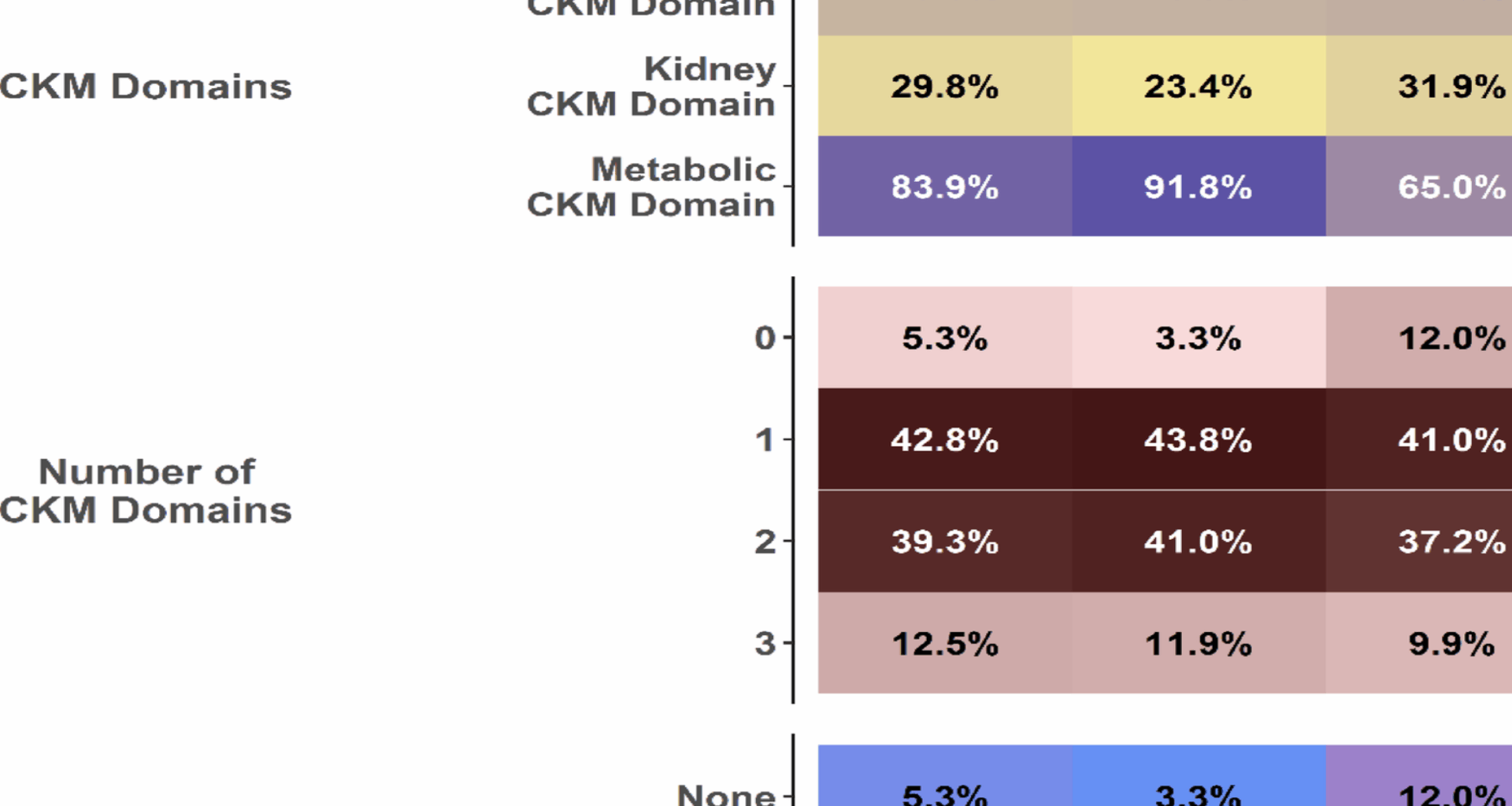
Numbers of CKM domains: either 0, 1, 2, or 3 domains
Groups of CKM: patients with none (0) domains of the CKM syndrome; patients with only cardiovascular, kidney, or metabolic domain; patients with cardiovascular and kidney (cardio-kidney), cardiovascular and metabolic (cardio-metabolic) or kidney and metabolic (kidney-metabolic) domains; patients with all domains (cardio-kidney metabolic).
To analyze the associations of the number of CKM domains and CKM groups with the management of patients with AF, we considered treatments received at baseline. We considered antithrombotic use (i.e. use of OAC and type of OAC, either a VKA or a non-vitamin K antagonist oral anticoagulant [NOAC]), other drugs (i.e., angiotensin converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARB], diuretics, beta-blockers [either selective or non-selective], digoxin, verapamil/diltiazem, propafenone, flecainide, amiodarone, dronedarone, other antiarrhythmic drugs, statins, insulin and oral hypoglycaemic agents) and interventional procedures (AF ablation and cardioversion), as collected at baseline.
Follow-up and outcomes
All patients recruited in the phase III of the GLORIA-AF registry underwent a 3-year follow-up, in which the incidence of major outcomes was recorded. For the purposes of this analysis, we evaluated the following outcomes, according to number of CKM domains, and CKM groups:
All-cause mortality;
Major adverse cardiovascular events (MACE, defined as the composite of cardiovascular death, stroke, and myocardial infarction);
Thromboembolism (defined as a composite of stroke, TIA and other, non-central nervous system thromboembolism);
Major Bleeding (defined as a life-threatening or fatal bleeding, symptomatic bleeding in a critical organ, or a bleeding associated with a haemoglobin reduction of ≥ 20 g/L or leading to ≥ 2 units of blood transfusion).
For this analysis, we considered the composite of all-cause death and MACE as our primary outcome, and evaluated the other outcomes as exploratory secondary outcomes.
Statistical analysis
Continuous variables were reported as either mean ± standard deviation (SD) or median and interquartile range [IQR], and were compared with parametric or non-parametric tests, respectively. Frequencies (percentages) were used to represent categorical variables, which were compared with chi-square test.
The association of geographical region of recruitment (i.e., Europe, North America, Asia or Latin America) with odds of presenting with each CKM domain was analyzed using multiple-adjusted logistic regression models. We defined a base set of covariates for adjustments, which included the components of the CHA2DS2-VASc score (i.e., age class [< 65, 65–75, or ≥ 75 years], sex, arterial hypertension, diabetes mellitus, CHF, CAD, PAD, and history of stroke/TIA), BMI, CrCl, type of AF (either paroxysmal, persistent or permanent), history of previous bleeding, and hyperlipidemia; then, for each CKM domain, we fitted a logistic regression model adjusted for all those covariates, except those used to define that domain (i.e., for the regression on cardiovascular domain, we excluded from covariates CHF, CAD, PAD and history of stroke/TIA; for the kidney domain, we excluded CrCl; for the metabolic domain, we excluded diabetes, BMI and hyperlipidemia). Results were reported as Odds Ratio (OR) and 95% Confidence Intervals (CI).
The odds of receiving an oral anticoagulant (OAC), and NOACs vs. VKAs in patients prescribed an OAC, were analyzed using multiple-adjusted logistic regression models. Covariates included the components of the CHA2DS2-VASc score, geographical region of recruitment, BMI, CrCl, type of AF, history of previous bleeding, and hyperlipidemia. Results were reported as OR and 95%CI.
For the primary composite outcome of all-cause death and MACE, we assessed Kaplan–Meier curves according to numbers of CKM domains and CKM groups, and compared survival distributions using log-rank test; p values were adjusted with Benjamini–Hochberg method. For groups of CKM, we represented survival curves in cardiovascular, kidney, and metabolic panels, to improve visualization.
For all outcomes we reported incidence rate (IR) per 100 patients/year and 95%CI, according to number of CKM domains and CKM groups. We also performed multiple-adjusted Cox regressions to evaluate the hazard of the primary and secondary outcomes. Covariates included were components of the CHA2DS2-VASc score, geographical region of recruitment, BMI, CrCl, type of AF, history of previous bleeding, hyperlipidemia and use of OAC at baseline. Results were reported as Hazard Ratio (HR) and 95%CI.
We finally performed a sensitivity analysis, by applying more restrictive definitions to the kidney and metabolic domains (i.e., CrCl < 30 ml/min instead of < 60 ml/min for the kidney domain; BMI ≥ 30 kg/m2 instead of ≥ 25 kg/m2, along with the other criteria, for the metabolic domain), and we evaluated the associations with the primary outcome according to the recalculated number of CKM domains and CKM groups.
A two-sided p < 0.05 was considered statistically significant. All analyses were performed using R 4.3.1 (R Core Team 2020, Vienna, Austria).