All animals, from jellyfish to humans, need sleep. But how these wide-ranging organisms control that need has remained a mystery.
It turns out that—in fruit flies, at least—sleep might be an “inescapable consequence” of aerobic metabolism, according to a new study. Mitochondria in Drosophila’s sleep-regulating neurons sense metabolic damage that accumulates during waking hours and trigger the pressure to sleep.
“It’s a really beautiful contribution,” says Keith Hengen, associate professor of biology at Washington University in St. Louis, who was not involved in the work. The study explains how the brain integrates information from a metabolic thermostat to regulate sleep pressure, Hengen says. “That’s a really hard problem, and I think they’ve nailed it.”
The regulators of sleep are distinct from the function of sleep, Hengen and other sleep researchers note. Just as fullness regulates food intake, but food intake doesn’t so much serve to fill the stomach as to get calories and nutrients, “we need to make this distinction between sensing of sleep pressure and the function of sleep,” says Giorgio Gilestro, associate professor of systems neurobiology at Imperial College London, who was not involved in the new study.
And with respect to sleep pressure, he adds, there are two processes at play: a well-studied circadian clock mechanism that links sleep to daylight cycles, and a less-understood homeostatic process that fine-tunes the need for sleep based on other factors.
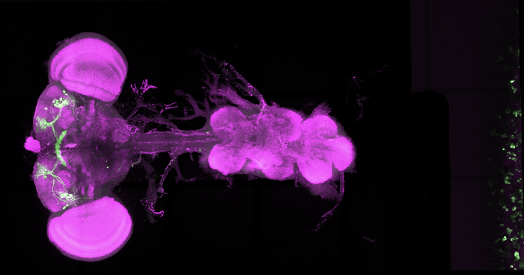
The new work focused on the latter, homing in on a set of specialized neurons in the fly brain that project to the dorsal fan-shaped body (dFBNs) and are distinct from those cells that regulate circadian rhythm.
M
itochondrial genes in dFBN neurons become activated in sleep-deprived flies, says Gero Miesenboeck, director of the Centre for Neural Circuits and Behaviour at the University of Oxford and the study’s principal investigator. “This is an absolutely amazing signature that really points to only a single sleep-need dependent process, and that is mitochondrial respiration.”
Mitochondria in flies that underwent 12 hours of sleep deprivation appeared fragmented, Miesenboek and his team discovered after dissecting the insects’ brains and imaging them under a confocal microscope. The fragments then ended up in the neuron’s lysosomes, where they were degraded and recycled. By contrast, in flies that were allowed 24 hours of recovery sleep after 12 hours of sleep deprivation, the mitochondria recovered, becoming large and branched again, similar to how they appeared in control flies that slept as much as they wanted. The findings were published in July in Nature.
We really think sleep does something very ancient and very primitive and very metabolic.
—
—
Gero Miesenboeck
Miesenboeck’s team inferred that sleepiness is related to mitochondrial damage that accumulates during wakefulness. Mitochondria produce energy by stripping electrons from fuel and transferring them along three protein complexes until they reach an oxygen and produce water—a transport chain that also pumps protons across the mitochondrial membrane through an enzyme to make adenosine triphosphate (ATP). If the number of electrons entering the transport chain and the number needed to fuel ATP production don’t match, however, electrons leak out and generate harmful reactive oxygen species.
In dFBN neurons, during wakefulness ATP demand is low, but electrons continue to flow into the transport chain, leading to a steady accumulation of reactive oxygen species, Miesenboeck’s team hypothesized. In turn, the proteins and lipids they damage activate signaling pathways that trigger the dFBN neurons to induce sleep.
To test this idea, the team decreased the rate of electron leakage in the neurons—either by introducing an enzyme that acts as a safety valve for electrons or by introducing a channel in the mitochondria membrane through which protons could flow. Both manipulations cut the need for sleep by about 50 percent—“a huge amount,” Miesenboeck says.
By contrast, activating the production of ATP via optogenetics caused the electrons to leak en masse—and triggered the flies to sleep.
These experiments suggest that sleep may be a consequence of inevitable electron leakage from the electron transport chain during respiration. “We really think sleep does something very ancient and very primitive and very metabolic,” Miesenboeck says. Other sleep functions, such as memory consolidation, might have evolved later, he adds.
“Definitely something huge is changing in the morphology of mitochondria” between the sleep-deprived animals compared with the controls, says Daniel Silverman, a postdoctoral fellow in Yang Dan’s laboratory at the University of California, Berkeley, who was not involved in the study. He described the differences as “striking.”
I
t would be interesting to see the activity of wake-promoting neurons, which could become fatigued during extended wakefulness, Silverman notes. It’s possible the dynamics of mitochondrial fission and fusion in these neurons could also contribute to the drive for recovery sleep, he says.
The experiments are rigorous and the findings convincing, Hengen says, but the mitochondrial changes are highly specific to this set of dFBN neurons. If the team’s metabolic theory for sleep is correct, mitochondrial reactive oxygen species accumulation should be a global, universal process across any brain tissue that is active during wakefulness, he says.
Perhaps this dFBN-based sensor prevents the accumulation of damage throughout the brain, Miesenboeck speculates: These neurons may experience a larger electron leak than other brain cells and behave like fuses that “blow first and put the animal to sleep before the brain gets damaged irreversibly … like a circuit breaker.”
Scientists debate whether sleep is regulated locally or in a more distributed way in different animals. In mammals, for example, there is evidence of “local sleep,” Gilestro says, in which some parts of the brain are asleep while others are awake, indicating that sleep pressure can be regulated in a distributed way rather than by a global switch like the one described in Meisenboeck’s study.
“I think the debate is still open,” Gilestro says.