It’s a fair question: atoms are made mostly of empty space, so why does solid matter feel, well… solid? As strange as it seems, two laws of physics stop us from walking through walls—and the reasons have nothing to do with magic.
Think of Vision in Avengers, or Harry Potter vanishing into Platform 9¾. Easy enough in fiction. But try it in the real world, and you’ll be greeted by a sore nose and a very stubborn wall.
What atoms are made of—and why it matters
Atoms are the building blocks of matter, and they’re surprisingly hollow. A tiny nucleus sits in the center—about 100,000 times smaller than the atom itself—while electrons orbit far away. So again, why do solid things feel so… solid?
Experts point to two fundamental principles: electrostatic repulsion and the Pauli exclusion principle.
In a classical view, electrons orbit the nucleus like planets around the sun. But in quantum physics, they don’t follow tidy paths. Instead, they form a “probability cloud”—a fuzzy region where they’re likely to be found. “It just sits there,” said Raheem Hashmani, a physics PhD student at the University of Wisconsin–Madison. “It shows where the electron is most likely to be.”
This electron cloud gives the atom a negative electric charge around its outer edges. So if you try to walk into a wall, your atoms and the wall’s atoms repel each other—just like trying to push two like poles of magnets together, explained physicist Steven Rolston of the University of Maryland.
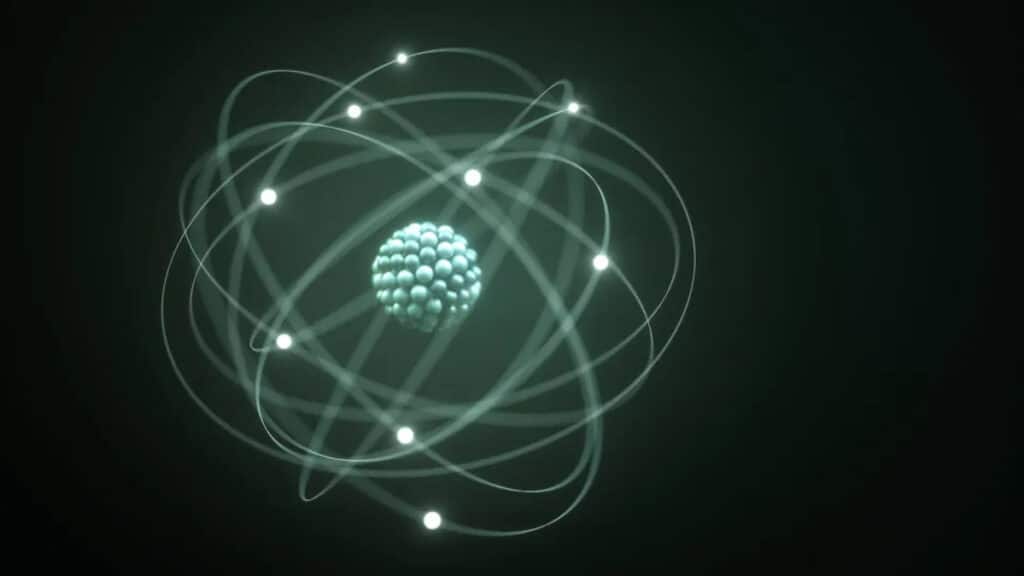
Atoms have a central nucleus surrounded by a “probability cloud” of electrons. (Image credit: KTSDesign/SCIENCEPHOTOLIBRARY via Getty Images)
Forces that keep solid matter solid
This electromagnetic repulsion keeps atoms from overlapping. Electrons don’t just bounce—they interact through electromagnetic waves. These invisible forces are why solid objects don’t pass through each other.
But what if you did manage to get atoms closer together?
Enter the Pauli exclusion principle. It says fermions—particles like electrons—can’t occupy the same space at the same time. “When the electron clouds overlap, it means two electrons might share the same space,” Hashmani said. “The Pauli principle doesn’t allow that.”
These two forces—Pauli exclusion and electromagnetic repulsion—prevent atoms from cramming into one another. Without them, solids would collapse. Even in liquids and gases, where atoms can move more freely, the same principles apply: atoms can move, but they can’t overlap.
A quantum exception: tunneling through barriers
And yet… quantum mechanics always has a twist.
Particles like electrons act like waves, not little balls. And sometimes, those waves tunnel through barriers.
Picture a wave hitting a wall. In classical physics, it’d just bounce off. But quantum waves decay slowly when they hit a barrier. If the wall is thin enough, a bit of the wave might still sneak through to the other side. That means there’s a slim—but real—chance the particle could appear beyond the wall. This is quantum tunneling.
But don’t get any ideas.
The odds of an entire person quantum tunneling through a wall? “Roughly one in 10 to the 10 to the 30,” said Hashmani. “Punch that into a calculator and you’ll just get zero. That’s how tiny the probability is.”
Rolston put it another way: “It’s about as close to zero as you can get—but it’s still not zero. I’m confident it wouldn’t happen in the lifetime of the universe.”