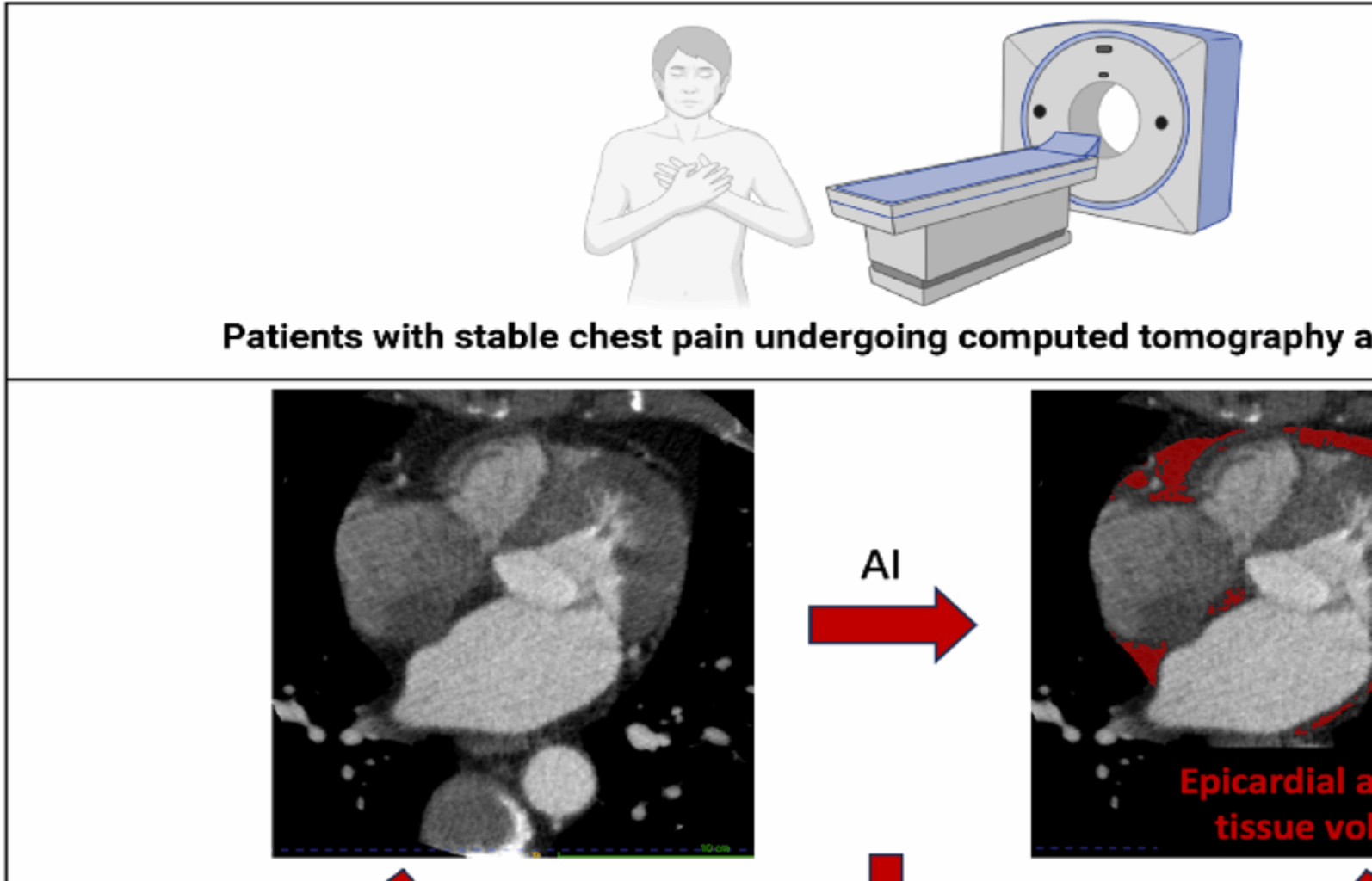
In this post-hoc analysis of a prospective multicenter study among patients presenting with stable chest pain, we report the association of fully automated deep learning measurements of epicardial adipose tissue volume with coronary plaque characteristics and the future risk of myocardial infarction. We showed that epicardial adipose tissue volume is correlated with quantitative plaque characteristics, and that this association was independent of body size related variations. Moreover, increased epicardial adipose tissue volume was observed in patients with cardiometabolic disease, and in this subpopulation, epicardial adipose tissue volume was an independent predictor of future risk of myocardial infarction, over and above established markers of cardiovascular risk, including body-mass index, cardiovascular risk score, coronary artery calcium score, coronary artery stenosis severity, low-attenuation plaque burden, and peri-coronary adipose tissue attenuation. Assessment of epicardial adipose tissue volume could therefore provide additional information to aid risk stratification of patients undergoing CT (Central Illustration).
Several previous studies have suggested an association between epicardial adipose tissue volume and the extent and severity of coronary artery disease [29,30,31]. This hypothesis is supported by our study which demonstrated an association between epicardial adipose tissue volume and coronary artery calcium score, presence of obstructive disease, and high-risk plaque characteristics. In addition, we showed an association between total, non-calcified, calcified, and low-attenuation plaque burden and peri-coronary adipose tissue attenuation, and this association was independent of body size related variations. Mechanistic studies are required to assess the etiology of the association between epicardial adipose tissue accumulation and coronary artery disease.
In our study, we demonstrated that epicardial adipose tissue volume independently predicts the risk of future myocardial infarction in patients with cardiometabolic disease. Several previous studies have looked at the ability of epicardial adipose tissue volume to predict major adverse cardiovascular events. In an observational study of 843 human immunodeficiency virus-infected patients, both the upper tertile of epicardial adipose tissue volume and coronary artery calcium score were independent predictors of coronary artery disease and all-cause mortality after adjusting for age and sex [32]. A large population-based study of 4093 patients free of cardiovascular disease showed that doubling of epicardial adipose tissue volume was associated with 1.5-fold risk of coronary events during follow-up period of 8 years and after adjustment for cardiovascular risk factors [33]. However, the incremental improvement by epicardial adipose tissue above cardiovascular risk factors alone was not significant. A recent meta-analysis concluded that epicardial adipose tissue volume assessed by CT is associated with higher risks of major adverse cardiovascular events including cardiac death, myocardial infarction, unstable angina, coronary revascularization, ischemic stroke, or heart failure [34]. West et al., developed a deep-learning network to quantify epicardial adipose tissue from CTA in the ORFAN study, and afterwards applied this to the SCOT-HEART patients [35]. They concluded that epicardial adipose tissue volume was able to predict the composite endpoint of all-cause mortality, myocardial infarction, and stroke, independent of clinical risk factors. Importantly, their results were not adjusted for qualitative and quantitative high-risk plaque features that can also be obtained from CT and did not consider only myocardial infarction as an outcome. Further, they did not consider analysis in patients with cardiometabolic disease, which is increasingly prevalent globally. In contrast, in our study we have evaluated the added prognostic value of epicardial fat volume compared to these other known predictors of myocardial infarction.
Cardiometabolic disease is characterized by a combination of cardiometabolic risk factors that lead to different symptoms but share a common etiology: obesity and insulin resistance. Their concomitant presence results in an increased risk of type 2 diabetes mellitus, coronary artery disease, and overall mortality [16]. Adipose tissue plays a central role in cardiometabolic disease, and is characterized by an increase in visceral adipose tissue, including epicardial adipose tissue. We found that patients with cardiometabolic disease had higher epicardial adipose tissue volume, and in this subgroup, epicardial adipose tissue volume was an independent predictor of future myocardial infarction. Although the predictive power was rather modest, epicardial adipose tissue may offer some value in risk assessment in this specific patient population. Chronic low-grade inflammation may be a potential important link between obesity, cardiometabolic disease, and adverse remodeling of epicardial fat, resulting in a proinflammatory phenotype [36]. In this proinflammatory state, epicardial adipose tissue can act as a local metabolic transducer allowing inflammatory mediators to influence the coronary vasculature directly and incite endothelial dysfunction and atherogenesis [37]. On the other hand, through the release of adipocytokines into the general circulation, epicardial adipose tissue in turn enhances the systemic inflammatory state linked to obesity [38]. Epicardial adipose tissue volume could therefore add different and complementary information to the already known predictors of myocardial infarction and can potentially be used as an additional imaging biomarker to further stratify risk in selected patients.
We observed higher epicardial adipose tissue attenuation, which is a measure of epicardial fat density, in patients with obstructive disease. There was a moderate correlation between epicardial adipose tissue attenuation and peri-coronary adipose tissue attenuation, yet no correlation with coronary artery calcium score or other high-risk plaque features. Furthermore, epicardial fat attenuation did not distinguish patients with increased risk of myocardial infarction during follow-up. While this is in line with previous studies demonstrating that epicardial adipose tissue volume rather than attenuation is associated with myocardial ischemia and coronary heart disease [32, 34, 36, 39, 40], other reports in different populations, including asymptomatic individuals [41] and patients with severe aortic stenosis undergoing aortic valve replacement [42], have shown significant associations between epicardial adipose tissue attenuation and adverse outcomes. These discrepancies likely reflect differences in population, CT acquisition, and outcome definitions. By contrast, we confirmed that peri-coronary adipose tissue attenuation is associated with future myocardial infarction [6], but only in patients without cardiometabolic disease; indicating that among patients with cardiometabolic disease, epicardial adipose tissue volume is the more important metric.
Automated quantification of epicardial adipose tissue volume could be readily incorporated into current coronary CT angiography workflows, as it can be derived directly from routine datasets without additional imaging, contrast, or radiation. With the increasing availability of AI-based tools for plaque analysis, integration of epicardial adipose tissue quantification would add minimal incremental cost or reporting time once validated software is available. This may enable clinicians to identify high-risk subgroups, particularly patients with cardiometabolic disease, who could benefit from more intensive preventive strategies.
Nonetheless, barriers to adoption remain. Wider implementation will depend on access to commercially available and regulatory-approved AI solutions, as well as further large-scale validation and demonstration of clinical utility. In addition, EAT quantification is not yet included in clinical guidelines, and evidence from prospective trials will be required before it can be formally incorporated into risk assessment algorithms.
This study has some limitations which should be acknowledged. This was a post-hoc analysis of previously collected data. We acknowledge that the sub-analysis of cardiometabolic disease patients only included a modest number of myocardial infarctions, and therefore, larger scale validation of the performance of epicardial adipose tissue volume as a risk predictor in this population is needed. Second, all participants in the SCOT-HEART trial were being assessed for suspected coronary artery disease and underwent CT angiography, which may limit the generalizability of our findings to populations without suspected coronary artery disease or without ready access to CT-based imaging. Finally, missing data were minimal, and patients with incomplete follow-up or uninterpretable CT angiography (e.g., due to significant motion artifact or poor contrast opacification) were excluded from the analysis; no imputation was performed as these exclusions were infrequent and evenly distributed across study groups.
In conclusion, automated epicardial adipose tissue volume measurements obtained from deep learning of CT angiography correlates with visual and quantitative plaque measures, and independently predicts the risk of fatal or non-fatal myocardial infarction in patients with cardiometabolic disease. Quantification of epicardial adipose tissue volume may offer some incremental enhancement in risk stratification in patients with cardiometabolic disease beyond currently known risk prediction tools and high-risk plaque features.