In line with the results of some earlier research, the clinical features of PTB patients with intestinal tuberculosis were linked to age, celiac disease, diarrhea, HGB, and ALB in our investigation [12, 16]. The nomogram provides a practical tool for risk assessment, and the identified cutoff value (−0.309) may assist in stratifying patients in clinical settings. By calculating an individual’s total points on the nomogram and comparing it to the cutoff, clinicians can identify those at higher risk of ITB who may benefit from further diagnostic evaluation. Patients with gastrointestinal symptoms during hospitalization require a high index of suspicion for intestinal tuberculosis. This is consistent with clinical practice. Patients under 60 years old with pulmonary tuberculosis have a greater probability to get intestinal tuberculosis, and TB prevalence has been found to be closely related to age [1].
In our study, albumin and hemoglobin levels at admission are independent predictors of intestinal tuberculosis in patients with PTB. The serum albumin concentration is an important marker of nutritional status [17]. Anemia was utilized as an indirect evaluation of the individuals’ nutritional status [18]. TB and malnutrition tend to correlate strongly with each other [19].
As previous studies have found, the relationship between tuberculosis and malnutrition is bidirectional, as malnutrition can also come from tuberculosis itself, which is caused by inflammation-related cachexia, anorexia, and malabsorption. Body mass index (BMI) is an important indicator of chronic malnutrition in the body, and determines whether an individual is obese and wasting. There is a negative correlation between the incidence of tuberculosis and BMI. People with mild, moderate and severe thinness had a 2.0-fold, 2.5-fold and 2.8-fold increased risk of TB compared to normal-weight subjects [20]. The daily dietary intake level is associated with the development of malnutrition in tuberculosis patients, and the intake of fruits and vegetables, poultry meat, fish and shrimp and milk in most tuberculosis patients does not reach the lowest intake level in China. Therefore, a lack of a proper diet may accelerate the progression of TB.
A series of animal investigations indicated that hypoalbuminemia could change the absolute and relative numbers of total T-lymphocytes and other immune system cell subpopulation [21], compromising host protection against MTB [22]. Bishlawy and IM EL shown that hemoglobin is highly bacteriostatic. In their experiment, a drop of washed RBCs is placed in a Petri dish containing nutritional agar injected with staphylococci and cultured for 24–48 h at 37 °C. The RBCs utilized were either pure or 50% diluted in saline [23]. The results demonstrated that undiluted washed RBCs inhibited bacterial growth but became impaired by dilution. This supports the conclusion that the incidence of intestinal tuberculosis is higher in anemic PTB patients than in non-anemic PTB patients, as well as why it was higher in moderate and severe anemic PTB patients compared to mild anemic patients.
To summarize, this may be the reason why PTB patients with anemia and hypoproteinemia are more likely to have intestinal tuberculosis.
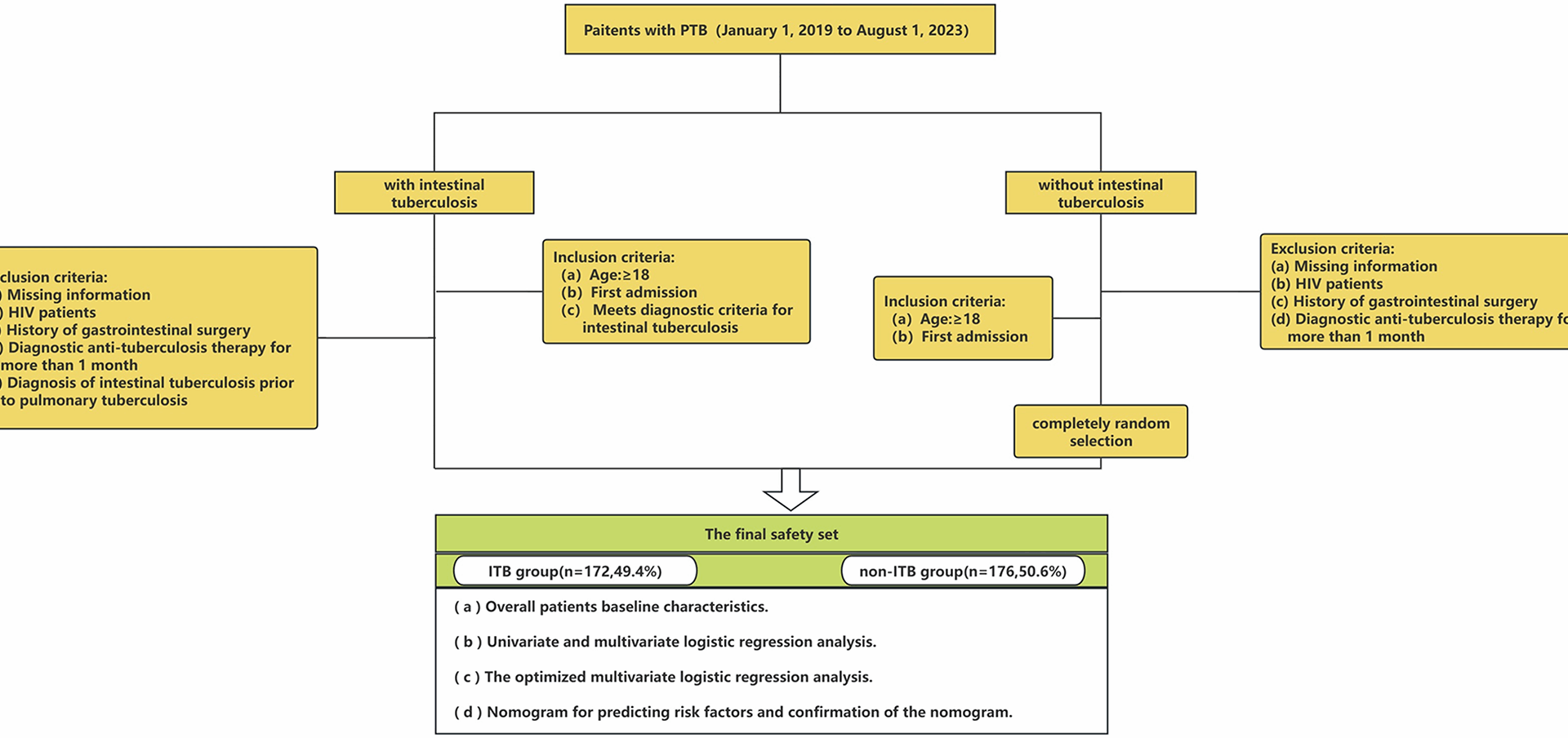
When people’s immune systems deteriorate, they become more vulnerable to PTB and extrapulmonary tuberculosis. For PTB patients, if intestinal tuberculosis is discovered early and effectively treated, major complications such as blockage, fistula formation, and perforation can be avoided [24]. ITB is recognized as one of the most common sites of extrapulmonary involvement. Endoscopy is an important tool for diagnosing ITB. However, hospitals in least developed country often lack gastrointestinal endoscopes, making it easy to miss patients with intestinal tuberculosis. As a result, it is necessary to take intestinal tuberculosis and PTB coinfection into account. An accurate prediction model is critical for both doctors and PTB patients. This helps doctors to identify potential patients with intestinal tuberculosis in time and improve the diagnosis rate of intestinal tuberculosis.
Although various clinical criteria have been offered earlier, no matching model has been discovered that predicts the likelihood of combined intestinal tuberculosis in PTB patients. In light of these factors, a multivariable model based on readily available clinical data was created and internally verified. We hope that this model will reduce the rate of missed diagnosis of intestinal tuberculosis.
Limitations
However, our study has several limitations that should be considered. First, due to its single center retrospective design, the study is subject to inherent selection and information biases. All data were derived from a single center, which may limit the generalizability of the nomogram to other populations or clinical settings. Second, diagnostic challenges were present; some predictors relied on self-reported clinical symptoms, which are subject to recall bias and potential misrepresentation. Third, the sample size, though substantial, remains relatively small for developing a robust predictive model, which may affect the stability of the identified predictors. Fourth, diagnostic uncertainty exists due to the modest sensitivity of current methods for confirming intestinal tuberculosis, such as histology, culture, and Xpert-MTB/RIF, potentially leading to misclassification. Furthermore, due to symptoms such as abdominal pain and diarrhea becoming strong predictors in our models (with high odds ratios), any initial misclassification of diagnoses could be amplified, leading to an overestimation of the predictive strength of these symptom variables. Finally, the model was evaluated only through internal validation, which may result in overfitting and overly optimistic performance estimates. External validation in multi-center prospective cohorts is needed to better assess calibration, discriminative power, and overall applicability.