We conducted our study using the TriNetX Research Network, a database containing electronic health records (EHRs) from many different healthcare organizations [9]. For this analysis, we focused our investigation on U.S.-based healthcare organizations and data from 2004 to 2024, collected on April 8, 2024. This database is regularly updated and adheres to ISO 27001:2013 standards and the HIPAA Security Rule. Since the data are fully de-identified, local Institutional Review Board approval was not required. More information about TriNetX can be found https://trinetx.com/real-world-resources/publications/trinetx-publication-guidelines/.
Cohort formation and exclusion criteria
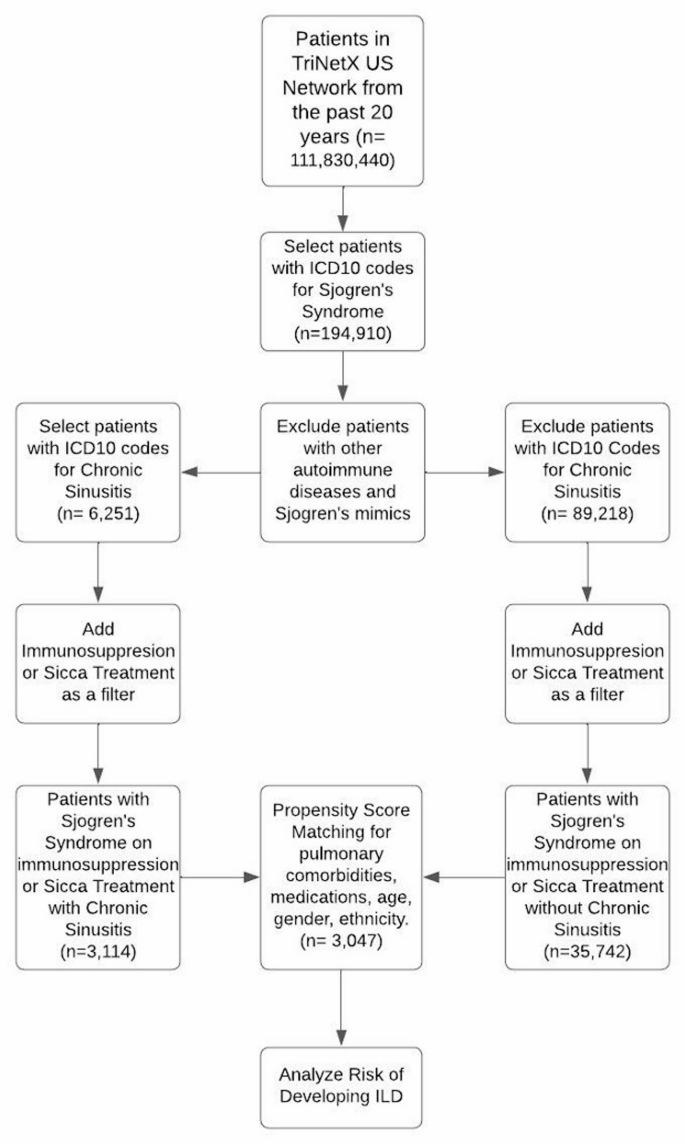
All cohorts were identified using ICD-10, RxNorm, TNX-curated, and Logical Observation Identifiers Names and Codes (LOINC) for diagnoses, medications, comorbidities, and laboratory tests (see Supplemental Table 1 for the list of codes). Inclusion criteria required patients to have a diagnosis of SS that preceded the diagnosis of CRS and ILD (Fig. 1). Additionally, patients had to be on either immunosuppressive medication or medications that treat mucocutaneous or ocular dryness.
Flowchart of cohort formation and analysis framework
To address potential confounding variables, we excluded patients diagnosed with any other immune-related disorders. These included systemic sclerosis (scleroderma), rheumatoid arthritis, dermatomyositis/polymyositis, systemic lupus erythematosus, Crohn’s disease, ulcerative colitis, giant cell arteritis, polymyalgia rheumatica, Takayasu arteritis, antineutrophil cytoplasmic antibody-associated vasculitis, sarcoidosis, multiple sclerosis, ankylosing spondylitis, and psoriasis. Additionally, patients with conditions associated with glandular dysfunction were excluded, such as graft-versus-host disease, human immunodeficiency virus infection, hepatitis C, IgG4-related disease, and those who had undergone organ transplantation.
Index event definition and time window for primary outcome
The index event was defined as the initial documentation of SS in the electronic health records. This study included two distinct cohorts. The first cohort comprised patients diagnosed with SS who subsequently developed CRS at least one day to five years after their SS diagnosis. The second cohort consisted of patients diagnosed with SS who did not have a diagnosis of CRS at any time during the study period. Patients with a diagnosis of CRS prior to their SS diagnosis were excluded from both cohorts. This exclusion ensured that CRS developed after the onset of SS, allowing us to specifically examine whether new onset CRS is a risk factor for the development of ILD.
Outcomes
The primary outcome of our study was the incidence of a new diagnosis of ILD. ILD cases were identified using ICD-10 codes for pulmonary fibrosis, Sjögren’s syndrome with lung involvement, progressive fibrotic phenotype, and respiratory bronchiolitis-associated interstitial lung disease.
Propensity Score Matching (PSM)
After defining cohorts, index events, outcomes, we adjusted results for relevant patient covariates. A logistic regression model was used to predict the probability (propensity score) of each patient belonging to cohorts based on their covariates. Using greedy nearest-neighbor matching with a caliper of 0.1 pooled standard deviations, the system matched patients from the smaller cohort with those in the larger cohort who had similar propensity scores.
The covariates used for PSM included age, race, gender, and body mass index (BMI). We also adjusted for various pulmonary conditions associated with airway symptoms, such as asthma, chronic obstructive pulmonary disease, and nicotine dependence (smoking). Additionally, we accounted for medications known to cause ILD, including nitrofurantoin, amiodarone, and bleomycin. Furthermore, cohorts were matched for immunosuppressive medications used to treat active Sjögren’s disease, such as methotrexate, azathioprine, mycophenolate, hydroxychloroquine, leflunomide, rituximab, sulfasalazine, tumor necrosis factor tumor necrosis factor-alpha inhibitors, and cyclophosphamide, as well as medications treating sicca symptoms, such as cyclosporine, pilocarpine, cevimeline, and lifitegrast.
Statistical analysis
All statistical analyses were performed using the TriNetX Advanced Analytics Platform. The precise methodologies for these computations are proprietary and protected under trade secret laws. This platform enabled us to calculate measures of association, including risk difference, risk ratio, and odds ratio, all with 95% confidence intervals and a significance threshold of p < 0.05 (two-sided).